Timing of Antenatal Corticosteroid Administration and Neonatal Outcomes
- PMID: 40388165
- PMCID: PMC12090034
- DOI: 10.1001/jamanetworkopen.2025.11315
Timing of Antenatal Corticosteroid Administration and Neonatal Outcomes
Abstract
Importance: Antenatal corticosteroids (ACS) are accepted to be most effective in reducing prematurity-related neonatal mortality and morbidity when administered 1 to 7 days before birth. However, precise data on the optimal timing of administration are scarce.
Objective: To investigate the association between ACS administration to birth interval as a continuous variable and neonatal outcomes among preterm neonates.
Design, setting, and participants: This national retrospective cohort study was conducted from 2018 to 2021 at level III neonatal intensive care units participating in the Canadian Neonatal Network. Participants included singleton and twin neonates born from 23 weeks 0 days' to 31 weeks 6 days' gestation. Data were analyzed from November 29, 2023, to March 8, 2024.
Exposure: ACS administration to birth interval.
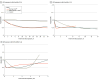
Main outcomes and measures: The primary outcome was neonatal mortality. The secondary outcome was a composite of mortality or severe neurologic injury. Associations of the ACS administration to birth interval with the study outcomes were modeled using restricted cubic splines with 5 knots.
Results: A total of 7950 neonates met the study criteria, from 7124 pregnancies (mean [SD] maternal age, 31.1 [5.7] years). Compared with individuals who received ACS, those who did not were younger and had lower rates of nulliparity, twin pregnancy, hypertension, and gestational diabetes. The overall rates of neonatal mortality and the composite outcome were 8% (670 of 7950) and 14% (1132 of 7950), respectively. ACS exposure was associated with reduced neonatal mortality as early as 2 hours after administration (adjusted risk ratio [ARR], 0.83 [95% CI, 0.70-1.00]). The reduction in mortality risk increased to a plateau 12 hours following exposure (ARR, 0.56 [95% CI, 0.40-0.78]), remained stable for the first 2 weeks following exposure, and gradually decreased after that. The association with reduced mortality was no longer observed at 4 weeks after administration (ARR, 0.82 [95% CI, 0.56-1.20]), and the ARR approached the null 5 weeks after administration (ARR, 0.99 [95% CI, 0.56-1.73]). This pattern was not affected by gestational age at birth or the number of fetuses.
Conclusions and relevance: In this cohort of neonates born from 23 weeks 0 days' gestation to 31 weeks 6 days' gestation, ACS administration was associated with a reduction in neonatal mortality as early as 2 hours after the administration of the first dose. The interval associated with the greatest reduction in neonatal mortality was between 12 hours and 14 days before birth, which was wider than the currently accepted optimal interval of 1 to 7 days. These findings may have important clinical implications for the management of pregnancies at risk of preterm birth, particularly regarding the administration of ACS even in cases in which preterm birth is imminent, and the timing of repeat courses of ACS.
Conflict of interest statement
Figures


References
-
- Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12(2):1-24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

