Visceral leishmaniasis in kidney transplant recipients and candidates: an integrative review of the last 20 years
- PMID: 40388290
- PMCID: PMC12088644
- DOI: 10.1590/2175-8239-JBN-2024-0138en
Visceral leishmaniasis in kidney transplant recipients and candidates: an integrative review of the last 20 years
Abstract
Introduction: Leishmaniasis is a potential concern for solid organ transplant (SOT) recipients, particularly those from endemic regions. Among SOT procedures, kidney transplantation (KT) is the most common. This study aims to synthesize the evidence about visceral leishmaniasis (VL) in KT candidates and recipients, with a focus on risk factors and associated outcomes.
Methods: This integrative review analyzed studies from the past 20 years, focusing on disease profile, treatment, prognosis, and risk of asymptomatic infection.
Results: A total of 32 articles were included. Of the KT recipients, 85.7% were male, with an average age of 42.5 years. The average timespan since symptom onset was 54.7 months. Renal function impairment was reported in 64% of patients, with an associated mortality rate of 15%. Post-treatment relapse occurred in 10-37.5% of patients. Among KT candidates, 13.9% were seropositive for Leishmania spp.
Conclusion: VL is an infrequent condition among KT recipients, limiting the quality of the available evidence. Early detection and prompt treatment are crucial for improving outcomes. While renal function impairment is common, it rarely leads to graft rejection. In the reviewed studies, the coexistence of VL and cutaneous or mucocutaneous forms was linked to higher mortality. Recurrences are common and require individualized management strategies. Hemotransfusion poses a potential infection risk, although routine screening in blood banks is not yet recommended.
Introdução:: A leishmaniose é uma possível preocupação para receptores de transplante de órgãos sólidos (TOS), especialmente aqueles provenientes de regiões endêmicas. Dentre os procedimentos de TOS, o transplante renal (TR) é o mais comum. Este estudo tem como objetivo sintetizar as evidências sobre leishmaniose visceral (LV) em candidatos e receptores de TR, com foco nos fatores de risco e desfechos associados.
Metodos:: Esta revisão integrativa analisou estudos dos últimos 20 anos, concentrando-se no perfil da doença, tratamento, prognóstico e risco de infecção assintomática.
Resultados:: Foram incluídos 32 artigos. Dos receptores de TR, 85,7% eram do sexo masculino, com idade média de 42,5 anos. O tempo médio desde o início dos sintomas foi de 54,7 meses. O comprometimento da função renal foi relatado em 64% dos pacientes, com uma taxa de mortalidade associada de 15%. A recidiva pós-tratamento ocorreu em 10-37,5% dos pacientes. Entre os candidatos ao TR, 13,9% apresentaram soropositividade para Leishmania spp.
Conclusão:: A LV é uma condição pouco frequente entre receptores de TR, o que limita a qualidade das evidências disponíveis. A detecção precoce e o tratamento imediato são cruciais para a melhoria dos desfechos. Embora o comprometimento da função renal seja comum, ele raramente leva à rejeição do enxerto. Nos estudos revisados, a coexistência de LV e formas cutâneas ou mucocutâneas esteve associada a maior mortalidade. As recidivas são comuns e exigem estratégias de manejo individualizadas. A hemotransfusão representa um potencial risco de infecção, embora a triagem de rotina em bancos de sangue ainda não seja recomendada.
Conflict of interest statement
Figures

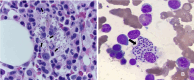




References
-
- Favi E, Santolamazza G, Botticelli F, Alfieri C, Delbue S, Cacciola R, et al. Epidemiology, clinical characteristics, diagnostic work up, and treatment options of leishmania infection in kidney transplant recipients: a systematic review. Trop Med Infect Dis. 2022;7(10):258. doi: 10.3390/tropicalmed7100258. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

