Metal-Catalyzed Fluoroacetyl Carbene Transfer from Sulfonium Salts
- PMID: 40388296
- PMCID: PMC12131225
- DOI: 10.1021/acs.orglett.5c01416
Metal-Catalyzed Fluoroacetyl Carbene Transfer from Sulfonium Salts
Abstract
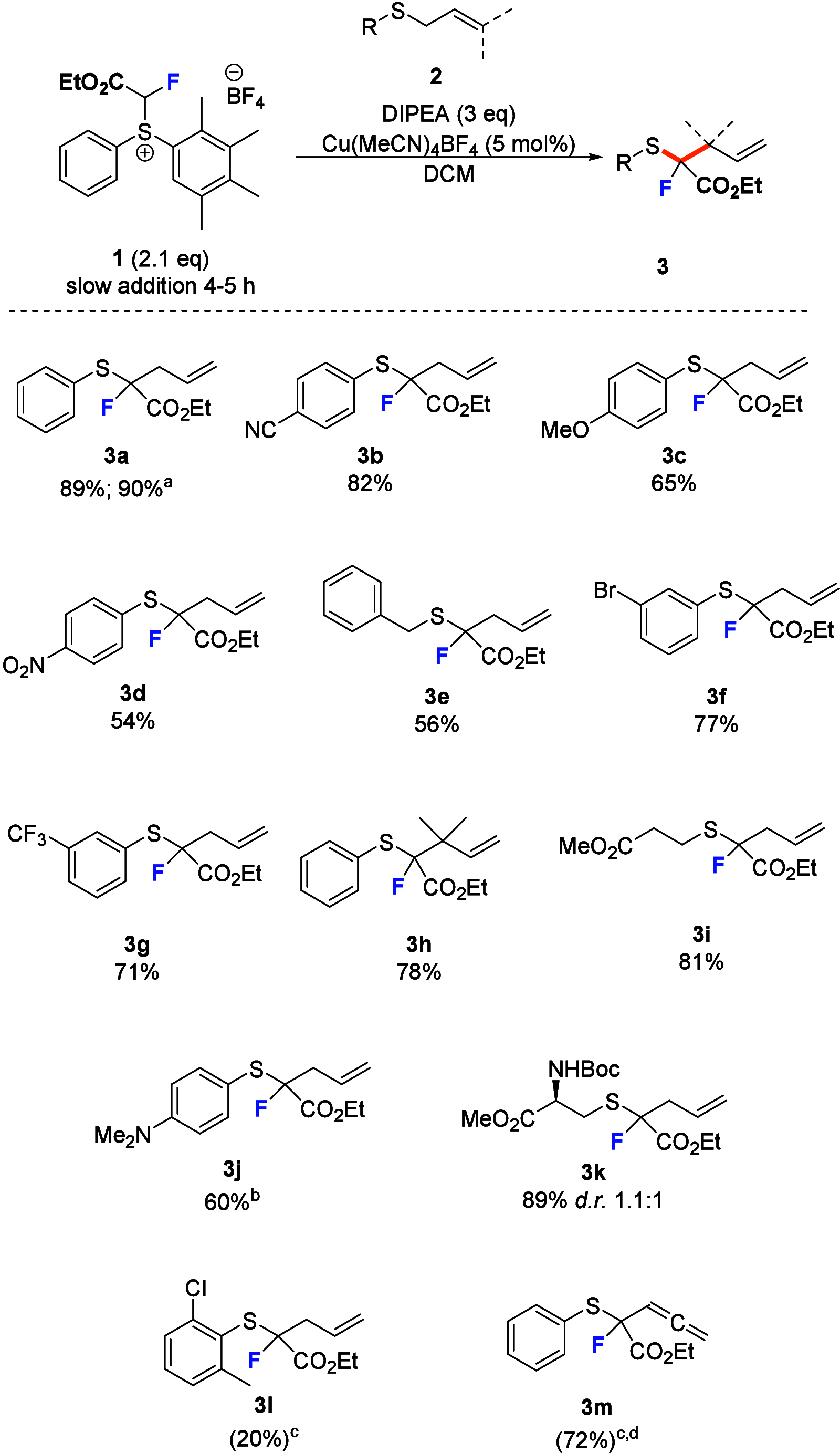
The synthesis of organofluorine compounds is pivotal in developing innovative pharmaceuticals, materials, and agrochemicals. Fluorinated carbene transfer offers a promising strategy for forming new carbon-carbon and carbon-heteroatom bonds, facilitating one-carbon editing by simultaneously introducing fluorine into target structures. In this study, we introduce a novel fluoroacetyl sulfonium reagent, (2-ethoxy-1-fluoro-2-oxoethyl)(phenyl)(2,3,4,5-tetramethylphenyl)sulfonium tetrafluoroborate (1), serving as an effective alternative to the currently unknown 2-diazo-2-fluoroacetate for ethyl fluoroacetyl carbene transfer. This reagent is applied in Doyle-Kirmse and cyclopropanation reactions operating under mild conditions with the use of earth-abundant metal catalysts. This approach enables the efficient synthesis of valuable monofluorinated products.
Figures



Similar articles
-
Copper-Catalyzed Enantioselective Doyle-Kirmse Reaction of Azide-Ynamides via α-Imino Copper Carbenes.Angew Chem Int Ed Engl. 2023 Mar 1;62(10):e202216923. doi: 10.1002/anie.202216923. Epub 2023 Jan 25. Angew Chem Int Ed Engl. 2023. PMID: 36639865
-
Chiral Nickel(II) Complex Catalyzed Enantioselective Doyle-Kirmse Reaction of α-Diazo Pyrazoleamides.J Am Chem Soc. 2018 Mar 7;140(9):3299-3305. doi: 10.1021/jacs.7b12486. Epub 2018 Feb 22. J Am Chem Soc. 2018. PMID: 29444400
-
Recent advances in metalloporphyrin-catalyzed fluorinated carbene transfer reactions.Chem Commun (Camb). 2025 Sep 11;61(74):13988-14001. doi: 10.1039/d5cc03443d. Chem Commun (Camb). 2025. PMID: 40838314 Review.
-
Dimethoxyacetaldehyde-N-triftosylhydrazone: Preparation and Carbene Reactivity in Cyclopropanation and Doyle-Kirmse Reactions.Org Lett. 2025 Feb 28;27(8):1941-1948. doi: 10.1021/acs.orglett.5c00223. Epub 2025 Feb 20. Org Lett. 2025. PMID: 39976213
-
Triarylborane Catalyzed Carbene Transfer Reactions Using Diazo Precursors.ACS Catal. 2022 Jan 7;12(1):442-452. doi: 10.1021/acscatal.1c04746. Epub 2021 Dec 17. ACS Catal. 2022. PMID: 35028191 Free PMC article. Review.
References
-
- Zhou Y., Wang J., Gu Z., Wang S., Zhu W., Aceña J. L., Soloshonok V. A., Izawa K., Liu H.. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016;116:422–518. doi: 10.1021/acs.chemrev.5b00392. - DOI - PubMed
-
- Yuan T., Shi L.. Recent advances in carbon atom addition for ring-expanding single-atom skeletal editing. Organic Chemistry Frontiers. 2024;11:7318–7332. doi: 10.1039/D4QO01806K. - DOI
LinkOut - more resources
Full Text Sources

