Surfing in the storm: how Paraburkholderia xenovorans thrives under stress during biodegradation of toxic aromatic compounds and other stressors
- PMID: 40388301
- PMCID: PMC12117332
- DOI: 10.1093/femsre/fuaf021
Surfing in the storm: how Paraburkholderia xenovorans thrives under stress during biodegradation of toxic aromatic compounds and other stressors
Abstract
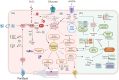
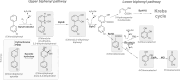
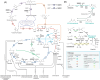
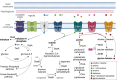
The adaptive mechanisms of Burkholderiales during the catabolism of aromatic compounds and abiotic stress are crucial for their fitness and performance. The aims of this report are to review the bacterial adaptation mechanisms to aromatic compounds, oxidative stress, and environmental stressful conditions, focusing on the model aromatic-degrading Paraburkholderia xenovorans LB400, other Burkholderiales, and relevant degrading bacteria. These mechanisms include (i) the stress response during aromatic degradation, (ii) the oxidative stress response to aromatic compounds, (iii) the metabolic adaptation to oxidative stress, (iv) the osmoadaptation to saline stress, (v) the synthesis of siderophore during iron limitation, (vi) the proteostasis network, which plays a crucial role in cellular function maintenance, and (vii) the modification of cellular membranes, morphology, and bacterial lifestyle. Remarkably, we include, for the first time, novel genomic analyses on proteostasis networks, carbon metabolism modulation, and the synthesis of stress-related molecules in P. xenovorans. We analyzed these metabolic features in silico to gain insights into the adaptive strategies of P. xenovorans to challenging environmental conditions. Understanding how to enhance bacterial stress responses can lead to the selection of more robust strains capable of thriving in polluted environments, which is critical for improving biodegradation and bioremediation strategies.
Keywords: Paraburkholderia; adaptation; aromatic compound; chaperone; proteostasis; stress.
© The Author(s) 2025. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

