Effect of Helicobacter pylori eradication on remnant stomach neoplasms after curative gastrectomy (HELP-GC): Protocol of a HELP-GC randomized controlled trial
- PMID: 40388525
- PMCID: PMC12088511
- DOI: 10.1371/journal.pone.0320903
Effect of Helicobacter pylori eradication on remnant stomach neoplasms after curative gastrectomy (HELP-GC): Protocol of a HELP-GC randomized controlled trial
Abstract
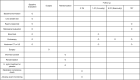
Background: Limited research has examined the direct effectiveness of Helicobacter pylori eradication (HPE) on the remnant stomach neoplasms after curative gastrectomy. This study aims to assess whether HPE could prevent the development of gastric neoplasms in the remnant stomach after curative gastrectomy through a double-blinded, randomized controlled trial.
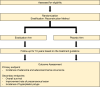
Methods: After curative gastrectomy, patients with HP infection and pathologically proven stage 1 tumors will be enrolled and randomly assigned to eradication (n = 492) and placebo (n = 492) groups. Patients in the eradication arm will be given the eradication regimen, which will comprise 40 mg of esomeprazole, 1 g of amoxicillin, and 500 mg of clarithromycin twice a day for 14 days. The primary endpoint is the development of gastric neoplasms, including adenoma or adenocarcinoma. The secondary endpoints are the 10-year overall survival, improvement rates of gastric glandular atrophy and/or intestinal metaplasia, and incidence of new-onset hyperplastic polyps among the groups.
Significance: This research will be the first randomized controlled clinical study in which a thorough long-term follow-up will be needed to evaluate the effectiveness of HPE for remnant stomach neoplasms after curative gastrectomy. Its results will serve as a basis for developing future strategies in the management of patients with HP infection who undergo curative gastrectomy.
Trial registration: https://cris.nih.go.kr/ KCT0008855. October 10, 2023.
Copyright: © 2025 Ko et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Effect of Helicobacter pylori Eradication on Long-Term Survival after Distal Gastrectomy for Gastric Cancer.Cancer Res Treat. 2016 Jul;48(3):1020-9. doi: 10.4143/crt.2015.264. Epub 2015 Nov 17. Cancer Res Treat. 2016. PMID: 26582396 Free PMC article.
-
Protocol for a randomised 'screen-and-treat' Helicobacter pylori eradication trial in 14-18-years-old adolescents residing in three regions of Chile: effectiveness and microbiological host implications.BMJ Open. 2025 Jan 30;15(1):e084984. doi: 10.1136/bmjopen-2024-084984. BMJ Open. 2025. PMID: 39890135 Free PMC article.
-
Efficacy and Safety of Polaprezinc-Based Therapy versus the Standard Triple Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Nutrients. 2022 Oct 4;14(19):4126. doi: 10.3390/nu14194126. Nutrients. 2022. PMID: 36235778 Free PMC article.
-
Systematic review and meta-analysis: proton pump inhibitor vs. ranitidine bismuth citrate plus two antibiotics in Helicobacter pylori eradication.Helicobacter. 2005 Jun;10(3):157-71. doi: 10.1111/j.1523-5378.2005.00307.x. Helicobacter. 2005. PMID: 15904473
-
Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis.Lancet Gastroenterol Hepatol. 2017 Oct;2(10):707-715. doi: 10.1016/S2468-1253(17)30219-4. Epub 2017 Aug 7. Lancet Gastroenterol Hepatol. 2017. PMID: 28781119
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous