Validation of Guidelines for Genetic Investigation of Myeloid Neoplasms with Germline Predisposition: Results from a Prospective Cohort Study
- PMID: 40388595
- PMCID: PMC12260513
- DOI: 10.1158/1078-0432.CCR-24-4251
Validation of Guidelines for Genetic Investigation of Myeloid Neoplasms with Germline Predisposition: Results from a Prospective Cohort Study
Abstract
Purpose: In a multicenter prospective cohort study, we assessed the diagnostic yield of the Nordic guidelines for germline investigation in myeloid neoplasms and mapped the spectrum of inherited and somatic variants.
Experimental design: Eighty-five patients (acute myeloid leukemia, n = 38; myelodysplastic syndromes, n = 26; thrombocytopenia, n = 14; and other, n = 7) fulfilling the Nordic criteria for germline investigation, based on (i) medical history or family history suggestive of a germline condition and (ii) relevant findings from the somatic diagnostic work-up (CytoMol), were recruited. The genetic analysis included enhanced whole-exome sequencing (n = 69) or sequencing of specific variants of interest (n = 16).
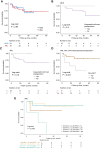
Results: Pathogenic or likely pathogenic (P/LP) germline variants were identified in 35% of patients (30/85). The diagnostic yield varied from 6% (1/16) in the family history group to 52% (17/33) in the CytoMol group. Germline DDX41 P/LP variants were the most frequent finding (13/30, 43% of all positive cases) almost exclusively found within the CytoMol group (12/13). Seven variants of unknown significance were also detected (TERT n = 2 and DDX41, RTEL1, ETV6, PARN, and SAMD9 n = 1). Five patients carried a P/LP variant in genes associated with another hereditary cancer syndrome (BRCA1 n = 3; PALB2 n = 1; and CHEK2; n = 1). Survival analysis showed a trend for longer survival among patients with acute myeloid leukemia and confirmed or suspected germline predisposition that underwent allogeneic stem cell transplantation.
Conclusions: The implementation of the Nordic guidelines in a prospective Swedish cohort results in a high overall diagnostic yield (35%), proving the feasibility and utility of these or similar guidelines in a clinical setting.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
B. Tesi reports grants from Region Stockholm (FoUI-985957), Swedish Society of Medicine (SLS-973171), and CIMED (FoUI-989107) during the conduct of the study. G. Barbany reports grants from Swedish Childhood Cancer Foundation (Barncancerfonden) during the conduct of the study. M. Jädersten reports other support from AbbVie, CanCell Therapeutics, AstraZeneca, and Pfizer outside the submitted work. No disclosures were reported by the other authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- 21 1823 Pj-BF1/Swedish Cancer Foundation
- PR2022-0135/Barncancerfonden (Swedish Childhood Cancer Foundation)
- PR2020-0128/Barncancerfonden (Swedish Childhood Cancer Foundation)
- 23 2976Fk/Swedish Cancer Foundation
- 20 1105Fk/Swedish Cancer Foundation
- FoUI-988468/Center for Innovative Medicine (CIMED)
- 24 0906 FK/Swedish Cancer Foundation
- 2020-01/Vleugels Stiftelse
- FoUI-985957/Stockholm läns landsting (Stockholm County Council)
- ALF-990591/Region Uppsala
- ALF-1002575/Region Uppsala
- ALF-991381/Region Uppsala
- SLS-973171/Svenska Läkaresällskapet (SLS)
- FoUI-989107/Center for Innovative Medicine (CIMED)
- 2022-1050146/Cancerfonden (Swedish Cancer Society)
- SLS-961271/Svenska Läkaresällskapet (SLS)
- 2023-1050180/Cancerfonden (Swedish Cancer Society)
- 23 2745 Pj/Swedish Cancer Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

