Imaging a concussion and the ensuing immune response at the blood-brain barrier
- PMID: 40388609
- PMCID: PMC12130861
- DOI: 10.1073/pnas.2414316122
Imaging a concussion and the ensuing immune response at the blood-brain barrier
Abstract
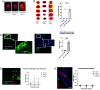
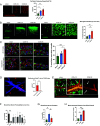
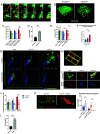
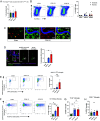
Concussions can cause debilitating symptoms despite no evidence of structural changes on diagnostic imaging. The cellular events occurring in the brain parenchyma following concussion, especially repetitive concussion, are not well elucidated. We developed a concussion model to induce a confined area of injury without causing frank hemorrhage. Using intravital microscopy, we observe activation of the vasculature that supported neutrophil rolling and platelet adhesion but no overt cellular recruitment from blood into brain parenchyma. Activated resident, not monocyte-derived, macrophages relocated to the injury site via Cx3cr1 and phagocytosed dysfunctional/detached astrocytes via scavenger receptors and TLR4, particularly after repetitive concussion. Additionally, microglia sealed areas of blood-brain barrier (BBB) disruption via purinergic pathways. Using a splitCre approach to dissect microglia and perivascular macrophages, we show that microglial invasion into the injury site is key to reducing BBB disruption. Our data suggest that microglia repair the BBB following concussion, but in doing so significantly alter the cellular ultrastructure of the brain milieu.
Keywords: astrocytes; blood–brain barrier; concussion; microglia; traumatic brain injury.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- Lulla A., et al. , Prehospital guidelines for the management of traumatic brain injury—3rd edition. Prehosp. Emerg. Care 27, 507–538 (2023). - PubMed
-
- Nguyen R., et al. , The international incidence of traumatic brain injury: A systematic review and meta-analysis. Can. J. Neurol. Sci. 43, 774–785 (2016). - PubMed
-
- Marmarou C. R., Walker S. A., Davis C. L., Povlishock J. T., Quantitative analysis of the relationship between intra- axonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury. J. Neurotrauma 22, 1066–1080 (2005). - PubMed
-
- Khatri N., et al. , Oxidative stress: Major threat in traumatic brain injury. CNS Neurol. Disord. Drug Targets 17, 689–695 (2018). - PubMed
-
- Patet C., Suys T., Carteron L., Oddo M., Cerebral lactate metabolism after traumatic brain injury. Curr. Neurol. Neurosci. Rep. 16, 31 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

