The GRAS protein RAM1 interacts with WRI transcription factors to regulate plant genes required for arbuscule development and function
- PMID: 40388617
- PMCID: PMC12130850
- DOI: 10.1073/pnas.2427021122
The GRAS protein RAM1 interacts with WRI transcription factors to regulate plant genes required for arbuscule development and function
Abstract
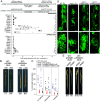
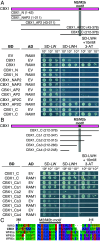
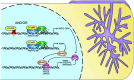
During arbuscular mycorrhiza (AM) symbiosis AM fungi form tree-shaped structures called arbuscules in root cortex cells of host plants. Arbuscules and their host cells are central for reciprocal nutrient exchange between the symbionts. REQUIRED FOR ARBUSCULAR MYCORRHIZATION1 (RAM1) encodes a GRAS protein crucial for transcriptionally regulating plant genes needed for arbuscule development and nutrient exchange. Similar to other GRAS proteins, RAM1 likely does not bind to DNA and how RAM1 activates its target promoters remained elusive. Here, we demonstrate that RAM1 interacts with five AM-induced APETALA 2 (AP2) transcription factors of the WRINKLED1-like family called CTTC MOTIF-BINDING TRANSCRIPTION FACTOR1 (CBX1), WRI3, WRI5a, WRI5b, and WRI5c via a C-terminal domain containing the M2/M2a motif. This motif is conserved and enriched in WRI proteins encoded by genomes of AM-competent plants. RAM1 together with any of these WRI proteins activates the promoters of genes required for symbiotic nutrient exchange, namely RAM2, STUNTED ARBUSCULES (STR), and PHOSPHATE TRANSPORTER 4 (PT4), in Nicotiana benthamiana leaves. This activation as well as target promoter induction in Lotus japonicus hairy roots depends on MYCS (MYCORRHIZA SEQUENCE)-elements and AW-boxes, previously identified as WRI-binding sites. The WRI genes are activated in two waves: Transcription of RAM1, CBX1, and WRI3 is coregulated by calcium- and calmodulin-dependent protein kinase-activated CYCLOPS, through the AMCYC-RE in their promoter, and DELLA, while WRI5a, b, and c promoters contain MYCS-elements and AW-boxes and can be activated by RAM1 heterocomplexes with CBX1 or WRI3. We propose that RAM1 provides an activation domain to DNA-binding WRI proteins to activate genes with central roles in AM development and function.
Keywords: GRAS transcription factor; arbuscular mycorrhiza; root; symbiosis; transcriptional regulation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- Smith S. E., Read D. J., Mycorrhizal Symbiosis (Academic Press, 2010).
-
- Roth R., Paszkowski U., Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 39, 50–56 (2017). - PubMed
-
- Keymer A., Gutjahr C., Cross-kingdom lipid transfer in arbuscular mycorrhiza symbiosis and beyond. Curr. Opin. Plant Biol. 44, 137–144 (2018). - PubMed
-
- Wang W., et al. , Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol. Plant 10, 1147–1158 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

