Histone Lactylation Antagonizes Senescence and Skeletal Muscle Aging by Modulating Aging-Related Pathways
- PMID: 40388671
- PMCID: PMC12165025
- DOI: 10.1002/advs.202412747
Histone Lactylation Antagonizes Senescence and Skeletal Muscle Aging by Modulating Aging-Related Pathways
Abstract
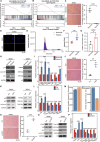
Epigenetic alterations are among the prominent drivers of cellular senescence and/or aging, intricately orchestrating gene expression programs during these processes. This study shows that histone lactylation, plays a pivotal role in counteracting senescence and mitigating dysfunctions of skeletal muscle in aged mice. Mechanistically, histone lactylation and lactyl-CoA levels markedly decrease during cellular senescence but are restored under hypoxic conditions primarily due to elevated glycolytic activity. The enrichment of histone lactylation at promoters is essential for sustaining the expression of genes involved in the cell cycle and DNA repair pathways. Furthermore, the modulation of enzymes crucial for histone lactylation, leads to reduced histone lactylation and accelerated cellular senescence. Consistently, the suppression of glycolysis and the depletion of histone lactylation are also observed during skeletal muscle aging. Modulating the enzymes can also lead to the loss of histone lactylation in skeletal muscle, downregulating DNA repair and proteostasis pathways and accelerating muscle aging. Running exercise increases histone lactylation, which in turn upregulate key genes in the DNA repair and proteostasis pathways. This study highlights the significant roles of histone lactylation in modulating cellular senescence as well as muscle aging, providing a promising avenue for antiaging intervention via metabolic manipulation.
Keywords: epigenetics; histone lactylation; senescence; skeletal muscle aging.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Lopez‐Otin C., Blasco M. A., Partridge L., Serrano M., Kroemer G., Cell 2023, 186, 243. - PubMed
-
- Yang J. H., Hayano M., Griffin P. T., Amorim J. A., Bonkowski M. S., Apostolides J. K., Salfati E. L., Blanchette M., Munding E. M., Bhakta M., Chew Y. C., Guo W., Yang X., Maybury‐Lewis S., Tian X., Ross J. M., Coppotelli G., Meer M. V., Rogers‐Hammond R., Vera D. L., Lu Y. R., Pippin J. W., Creswell M. L., Dou Z., Xu C., Mitchell S. J., Das A., O'Connell B. L., Thakur S., Kane A. E., et al., Cell 2024, 187, 1312. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
