The protective efficacy of inactivated vaccine against hemorrhagic fever with renal syndrome: A meta-analysis
- PMID: 40388733
- PMCID: PMC12091670
- DOI: 10.1097/MD.0000000000042463
The protective efficacy of inactivated vaccine against hemorrhagic fever with renal syndrome: A meta-analysis
Abstract
Background: Hemorrhagic fever with renal syndrome (HFRS) is a kind of natural epidemic diseases with rodents as the main source of infection. The main clinical manifestations of HFRS are fever, hemorrhage, congestion, hypotensive shock and kidney damage. Some studies showed that vaccination populations in infected areas with inactivated vaccines can reduce the incidence of the disease, but there are variations in protection rates among these studies. The aim of this study is to systematically evaluate the protective effect of inactivated vaccines against HFRS.
Methods: Web of Science, PubMed, SinoMed, Proquest, China National Knowledge Infrastructure Database, Wanfang Database, VIP Database were searched from their inception to December 2024. Newcastle-Ottawa Scale (NOS) was used to assess the quality of evidence, and a random-effects meta-analysis was done to calculate pooled risk ratios for vaccination uptake. All the relevant data were analyzed by using STATA 15.0.
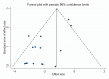
Results: A total of 15 articles were included, all of which explicitly reported the total number of vaccinated and unvaccinated people in the vaccination group, and the number of cases that developed during the observation period. Six of these articles reported positive antibody transfer rates. The protection rate of the inactivated HFRS vaccine reached 86%, and a subgroup analysis showed that there was a significant difference in the protection rate of the inactivated vaccine between Korea and China. The positive IgG antibody transfer rate was 97%, and neutralizing antibody transfer rate was 37%.
Conclusion: The results indicated that inactivated vaccine has a good protective effect against HFRS and should be universally administered to populations in high prevalence areas to control the harm caused by HFRS epidemics.
Keywords: inactivated vaccination; meta-analysis; protection rate; vaccine effectiveness.
Copyright © 2025 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures






Similar articles
-
[Comparative study on antibody levels of vaccination group and control group after 4 years of immunized with type B inactivated vaccine against hemorrhagic fever with renal syndrome].Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2001 Sep;15(3):274-6. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2001. PMID: 11986705 Clinical Trial. Chinese.
-
Long-term persistence of anti-hantavirus antibodies in sera of patients undergoing hemorrhagic fever with renal syndrome and subjects vaccinated against the disease.Infect Dis (Lond). 2016 Apr;48(4):262-266. doi: 10.3109/23744235.2015.1121289. Epub 2015 Dec 11. Infect Dis (Lond). 2016. PMID: 26654580
-
[Comparison of subclinical infection rates between vaccinated group with type I inactivated vaccine against hemorrhagic fever and controls].Zhonghua Yu Fang Yi Xue Za Zhi. 1999 Sep;33(5):308-10. Zhonghua Yu Fang Yi Xue Za Zhi. 1999. PMID: 11864500 Clinical Trial. Chinese.
-
Epidemiological progresses of hemorrhagic fever with renal syndrome in China.Chin Med J (Engl). 1999 May;112(5):472-7. Chin Med J (Engl). 1999. PMID: 11593522 Review.
-
Incidence rate of hemorrhagic fever with renal syndrome complicated with acute pancreatitis: a meta-analysis.Front Med (Lausanne). 2024 Oct 22;11:1442276. doi: 10.3389/fmed.2024.1442276. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39502643 Free PMC article.
References
-
- Matter S, Guzmán C, Figueiredo LT. Diagnosis of hantavirus infection in humans. Expert Rev Anti Infect Ther. 2015;13:939–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

