Sintilimab-induced diabetes mellitus and thyroid dysfunction in patient with gastric adenocarcinoma: A case report and literature review
- PMID: 40388765
- PMCID: PMC12091594
- DOI: 10.1097/MD.0000000000042490
Sintilimab-induced diabetes mellitus and thyroid dysfunction in patient with gastric adenocarcinoma: A case report and literature review
Abstract
Rationale: Immune checkpoint inhibitors bring hope to cancer patients but may also lead to severe immune-related adverse events (irAEs). Although irAEs during treatment are well-characterized, delayed immune-related events (DIRE) remain underreported. Here, we report a case of sintilimab-induced delayed immune-related diabetes mellitus, accompanied by ICI-related thyroid disease (ICI-TD). Cases involving both ICI-TD and ICI-related diabetes mellitus (ICI-DM) are also relatively rare. This study systematically aggregates dual endocrine irAEs to provide valuable insights for clinical practice.
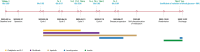
Patient concerns: A 60-year-old Chinese male diagnosed with gastric adenocarcinoma received a multimodal treatment regimen consisting of sintilimab, chemotherapy, and apatinib. He completed 3 cycles of chemotherapy and 4 cycles of sintilimab. Due to disease progression, sintilimab was discontinued, but apatinib was continued for an additional 1 month. No further antitumor therapy was administered afterward. Four months later, he was admitted to the emergency department due to persistent nausea, vomiting, and abdominal pain.
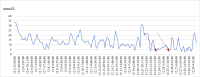
Diagnoses: Thyroid dysfunction induced by sintilimab was identified during treatment. His laboratory tests contributed to the diagnosis of diabetes ketoacidosis. Fulminant type 1 diabetes mellitus attributed to sintilimab met diagnostic criteria: plasma glucose 42.01 mmol/L, glycated hemoglobin 7.5%, C-peptide <0.02 µg/L, and negative islet autoantibodies.
Interventions: Levothyroxine replacement therapy was initiated for hypothyroidism, whereas diabetes ketoacidosis during hospitalization required intensive insulin therapy combined with fluid resuscitation.
Outcomes: The patient exhibited persistent blood glucose fluctuations during hospitalization, including 2 hypoglycemic episodes. Post-treatment stabilization required basal-bolus insulin at discharge, with continued levothyroxine for hypothyroidism.
Lessons: We report a rare case of concurrent ICI-TD and ICI-DM following sintilimab therapy. This case underscores the potential for DIRE, with onset occurring months posttreatment. Combined with a systematic review of existing cases, this study provides critical insights into surveillance strategies and pathogenesis of irAEs.
Keywords: DIRE; ICI-induced diabetes mellitus; ICI-induced thyroid dysfunction; irAEs; sintilimab.
Copyright © 2025 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
References
-
- Wu L, Tsang V, Menzies AM, et al. Risk factors and characteristics of checkpoint inhibitor-associated autoimmune diabetes mellitus (CIADM): a systematic review and delineation from type 1 diabetes. Diabetes Care. 2023;46:1292–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous