Dexmedetomidine- or Clonidine-Based Sedation Compared With Propofol in Critically Ill Patients: The A2B Randomized Clinical Trial
- PMID: 40388916
- PMCID: PMC12090071
- DOI: 10.1001/jama.2025.7200
Dexmedetomidine- or Clonidine-Based Sedation Compared With Propofol in Critically Ill Patients: The A2B Randomized Clinical Trial
Erratum in
-
Incorrect Values for Baseline Variables in Table 1.JAMA. 2026 Jan 6;335(1):94-95. doi: 10.1001/jama.2025.23530. JAMA. 2026. PMID: 41343186 Free PMC article. No abstract available.
Abstract
Importance: Whether α2-adrenergic receptor agonist-based sedation, compared with propofol-based sedation, reduces time to extubation in patients receiving mechanical ventilation in the intensive care unit (ICU) is uncertain.
Objective: To evaluate whether dexmedetomidine- or clonidine-based sedation reduces duration of mechanical ventilation compared with propofol-based sedation (usual care).
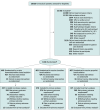
Design, setting, and participants: Pragmatic, open-label randomized clinical trial conducted at 41 ICUs in the UK including adults who were within 48 hours of starting mechanical ventilation, were receiving propofol plus an opioid for sedation and analgesia, and were expected to require mechanical ventilation for 48 hours or longer. The median time from intubation to randomization was 21.0 (IQR, 13.2-31.3) hours. Recruitment occurred from December 2018 to October 2023; the last follow-up occurred on December 10, 2023.
Interventions: The bedside algorithms used targeted a Richmond Agitation-Sedation Scale score of -2 to 1 (unless clinicians requested deeper sedation). The algorithms supported uptitration in the dexmedetomidine- and clonidine-based sedation intervention groups and supported downtitration for propofol-based sedation followed by sedation primarily with the allocated sedation (dexmedetomidine or clonidine). If required, supplemental use of propofol was permitted.
Main outcomes and measures: The primary outcome was time from randomization to successful extubation. The secondary outcomes included mortality, sedation quality, rates of delirium, and cardiovascular adverse events.
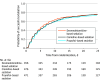
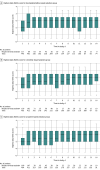
Results: Among the 1404 patients in the analysis population (mean age, 59.2 [SD, 14.9] years; 901 [64%] were male; and the mean APACHE II score was 20.3 [SD, 8.2]), the subdistribution hazard ratio (HR) for time to successful extubation was 1.09 (95% CI, 0.96-1.25; P = .20) for dexmedetomidine (n = 457) vs propofol (n = 471) and was 1.05 (95% CI, 0.95-1.17; P = .34) for clonidine (n = 476) vs propofol (n = 471). The median time from randomization to successful extubation was 136 (95% CI, 117-150) hours for dexmedetomidine, 146 (95% CI, 124-168) hours for clonidine, and 162 (95% CI, 136-170) hours for propofol. In the predefined subgroup analyses, there were no interactions with age, sepsis status, median Sequential Organ Failure Assessment score, or median delirium risk score. Among the secondary outcomes, agitation occurred at a higher rate with dexmedetomidine vs propofol (risk ratio [RR], 1.54 [95% CI, 1.21-1.97]) and with clonidine vs propofol (RR, 1.55 [95% CI, 1.22-1.97]). Compared with propofol, the rates of severe bradycardia (heart rate <50/min) were higher with dexmedetomidine (RR, 1.62 [95% CI, 1.36-1.93]) and clonidine (RR, 1.58 [95% CI, 1.33-1.88]). Compared with propofol, mortality was similar over 180 days for dexmedetomidine (HR, 0.98 [95% CI, 0.77-1.24]) and clonidine (HR, 1.04 [95% CI, 0.82-1.31]).
Conclusions and relevance: In critically ill patients, neither dexmedetomidine nor clonidine was superior to propofol in reducing time to successful extubation.
Trial registration: ClinicalTrials.gov Identifier: NCT03653832.
Conflict of interest statement
Figures

Comment in
-
The Search for the Single Best Sedative.JAMA. 2025 Jul 1;334(1):26-28. doi: 10.1001/jama.2025.7359. JAMA. 2025. PMID: 40388917 No abstract available.
References
-
- Maagaard M, Barbateskovic M, Andersen-Ranberg NC, et al. Dexmedetomidine for the prevention of delirium in adults admitted to the intensive care unit or post-operative care unit: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Acta Anaesthesiol Scand. 2023;67(4):382-411. doi: 10.1111/aas.14208 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

