Safety and broad immunogenicity of HIVconsvX conserved mosaic candidate T-cell vaccines vectored by ChAdOx1 and MVA in HIV-CORE 006: a double-blind, randomised, placebo-controlled phase 1 trial in healthy adults living without HIV-1 in eastern and southern Africa
- PMID: 40388952
- PMCID: PMC12134052
- DOI: 10.1016/j.lanmic.2024.101041
Safety and broad immunogenicity of HIVconsvX conserved mosaic candidate T-cell vaccines vectored by ChAdOx1 and MVA in HIV-CORE 006: a double-blind, randomised, placebo-controlled phase 1 trial in healthy adults living without HIV-1 in eastern and southern Africa
Abstract
Background: Even within the context of antiretroviral treatment and prevention, an HIV-1 vaccine remains the best strategy for ending the HIV/AIDS epidemic. A vaccine is particularly needed in sub-Saharan Africa, where HIV-1 greatly affects people's lives and economy. Here, we aimed to assess the safety and immunogenicity of candidate T-cell vaccines in African populations.
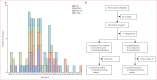
Methods: HIV-CORE 006 was a double-blind, randomised, placebo-controlled phase 1 trial conducted across four clinical research centres in Uganda, Kenya, and Zambia. Eligible participants were not pregnant, were living without HIV-1 or HIV-2, had a low likelihood of acquiring HIV-1, were aged 18-50 years, fully comprehended the purpose and details of this study as outlined in the participant information sheet, and passed an assessment of understanding before providing written informed consent. Participants were randomly assigned (9:2) to receive either a vaccine regimen or a placebo. The vaccine was administered as ChAdOx1.tHIVconsv1 (C1) followed by MVA.tHIVconsv3 (M3) and MVA.tHIVconsv4 (M4) in regimen C1-M3M4. The first primary outcome was the vaccines' safety assessment, assessed in all participants who received at least one vaccine or placebo dose. The second primary outcome evaluated the C1-M3M4 regimen's induction of HIVconsvX-specific T-cell responses by assessing the proportion of vaccine recipients who responded to the vaccination, assessed in all participants who received all doses of vaccine or placebo as per protocol. This study is registered with ClinicalTrials.gov, NCT04553016, and the Pan-African Clinical Trials Registry PACTR202006495409011, and is now closed.
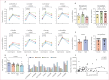
Findings: Between July 15, 2021, and Nov 2, 2022, 89 healthy adults living without HIV-1 were randomly assigned, with 88 receiving either the vaccine (n=72) or placebo (n=16). Of these 88 participants, 57 (65%) were male and 31 (35%) were female. The C1, M3, and M4 vaccine components were well tolerated and induced HIVconsvX-specific responses in 70 (99%) of the 71 participants who completed all vaccine doses. Vaccine-elicited T cells peaked at a median of 2310 (IQR 1080-4480) IFN-γ spot-forming units per 106 peripheral blood mononuclear cells and recognised a median of eight (five to ten) of ten peptide pools spanning the HIVconsvX immunogen. The total frequencies of elicited T cells decreased 4·6 times over a 40-week follow-up period compared with the peak responses. Upon antigenic re-exposure, T cells proliferated, exhibited multiple effector functions, and inhibited HIV-1 representatives from clades A, B, C, and D.
Interpretation: Results from key sub-Saharan African populations supported the safety of the vaccine regimen previously shown in the first-in-human trial in the UK. The induction of T cells and their characteristics encourage vaccine integration into HIV-1 cure strategies, which could inform HIV-1 prevention efforts.
Funding: The European and Developing Countries Clinical Trials Partnership.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests TH is a co-inventor of the HIVconsvX immunogens protected under EP14846993.5 and PCT/US14/58422 (WO2015048785). All other authors declare no competing interests.
Figures



References
-
- UNAIDS AIDS by the numbers. 2015. http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspubl...
-
- Bailón L, Llano A, Cedeño S, et al. Safety, immunogenicity and effect on viral rebound of HTI vaccines in early treated HIV-1 infection: a randomized, placebo-controlled phase 1 trial. Nat Med. 2022;28:2611–2621. - PubMed
-
- Hanke T. Aiming for protective T-cell responses: a focus on the first generation conserved-region HIVconsv vaccines in preventive and therapeutic clinical trials. Expert Rev Vaccines. 2019;18:1029–1041. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

