Characterization of a SARS-CoV-2 infection model in golden hamsters with diabetes mellitus
- PMID: 40389095
- PMCID: PMC12282415
- DOI: 10.1016/j.virs.2025.05.001
Characterization of a SARS-CoV-2 infection model in golden hamsters with diabetes mellitus
Abstract
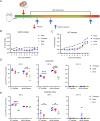
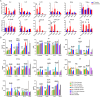
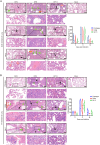
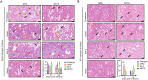
Being widespread across the globe, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) keeps evolving and generating new variants and continuously poses threat to public health, especially to the population with chronic comorbidities. Diabetes mellitus is one of high-risk factors for severe outcome of coronavirus disease 2019 (COVID-19). Establishment of animal models that parallel the clinical and pathological features of COVID-19 complicated with diabetes is thus highly essential. Here, in this study, we constructed leptin receptor gene knockout hamsters with the phenotype of diabetes mellitus (db/db), and revealed that the diabetic hamsters were more susceptible to SARS-CoV-2 and its variants than wild-type hamsters. SARS-CoV-2 and its variants induced a stronger immune cytokine response in the lungs of diabetic hamsters than in wild-type hamsters. Comparative histopathology analyses also showed that infection of SARS-CoV-2 and the variants caused more severe lung tissue injury in diabetic hamsters, and may induce serious complications such as diabetic kidney disease and cardiac lesions. Our findings demonstrated that despite the decreased respiratory pathogenicity, the SARS-CoV-2 variants were still capable of impairing other organs such as kidney and heart in diabetic hamsters, suggesting that the risk of evolving SARS-CoV-2 variants to diabetic patients should never be neglected. This hamster model may help better understand the pathogenesis mechanism of severe COVID-19 in patients with diabetes. It will also aid in development and testing of effective therapeutics and prophylactic treatments against SARS-CoV-2 variants among these high-risk populations.
Keywords: Diabetes; Hamster; Multiorgan injury; Pneumonia; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Copyright © 2025 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest All authors declare that there are no competing interests.
Figures


Similar articles
-
Kinetic Multi-omic Analysis of Responses to SARS-CoV-2 Infection in a Model of Severe COVID-19.J Virol. 2021 Sep 27;95(20):e0101021. doi: 10.1128/JVI.01010-21. Epub 2021 Jul 28. J Virol. 2021. PMID: 34319784 Free PMC article.
-
Marked reduction of SARS-CoV-2 infection and improved recovery following supplementation with a probiotic mix of four strains and two strains of Bifidobacterium breve in hamsters.Appl Environ Microbiol. 2025 Jun 18;91(6):e0064825. doi: 10.1128/aem.00648-25. Epub 2025 May 12. Appl Environ Microbiol. 2025. PMID: 40353655 Free PMC article.
-
Differential immunoregulation by human surfactant protein A variants determines severity of SARS-CoV-2-induced lung disease.Front Immunol. 2025 Apr 2;16:1462278. doi: 10.3389/fimmu.2025.1462278. eCollection 2025. Front Immunol. 2025. PMID: 40242753 Free PMC article.
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Alraddadi B.M., Watson J.T., Almarashi A., Abedi G.R., Turkistani A., Sadran M., Housa A., Almazroa M.A., Alraihan N., Banjar A., Albalawi E., Alhindi H., Choudhry A.J., Meiman J.G., Paczkowski M., Curns A., Mounts A., Feikin D.R., Marano N., Swerdlow D.L., Gerber S.I., Hajjeh R., Madani T.A. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi arabia, 2014. Emerg. Infect. Dis. 2016;22:49–55. - PMC - PubMed
-
- Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., Knighton P., Holman N., Khunti K., Sattar N., Wareham N.J., Young B., Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. - PMC - PubMed
-
- Buetti N., Trimboli P., Mazzuchelli T., Lo Priore E., Balmelli C., Trkola A., Conti M., Martinetti G., Elzi L., Ceschi A., Consonni V., Ogna A., Forni-Ogna V., Bernasconi E. Diabetes mellitus is a risk factor for prolonged SARS-CoV-2 viral shedding in lower respiratory tract samples of critically ill patients. Endocrine. 2020;70:454–460. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

