Engineering anti-BCMA CAR T cells for enhancing myeloma killing efficacy via apoptosis regulation
- PMID: 40389394
- PMCID: PMC12089368
- DOI: 10.1038/s41467-025-59818-8
Engineering anti-BCMA CAR T cells for enhancing myeloma killing efficacy via apoptosis regulation
Abstract
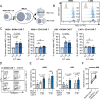
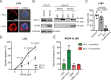
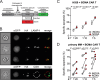
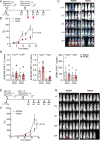
Clinical responses with chimeric antigen receptor (CAR) T cells are encouraging, but primary resistance and relapse after therapy prevent durable remission in many patients with cancer, with apoptosis resistance in cancer cells that limits killing by CAR T cells being a potential cause. Here we aim to boost tumor cell apoptosis induced by CAR T cells and find that anti-B cell maturation antigen (BCMA) CAR T cells over-expressing a granzyme B-NOXA fusion protein show improved killing of multiple myeloma (MM) cells in vitro and in xenograft mouse models in vivo. Mechanistically, such an enhancement is mediated by localizing NOXA to cytotoxic granules that are released into cancer cells upon contact. In MM cells, inhibition of MCL-1, an anti-apoptotic factor, by its natural ligand NOXA effectively induces apoptosis. Our data thus show that endowing granzyme B-NOXA expression to CAR T cells improves their killing efficacy, thereby presenting a potential generalizable enhancement for CAR T-mediated anti-cancer immunity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: V.P. received royalty payments related to venetoclax. V.P., Z.S., and T.K. are inventors on a patent for improving cytotoxicity of gene engineered T and NK cells. M.C.M. received research funding from Beigene, Janssen Cilag, Speakers fee from Janssen Cilag, Siemens, and Pfizer, and Consultancy fee from Janssen Cilag, Pfizer, BMS, and Hospitality Janssen Cilag, Beigene, all paid to institution. Z.S. and J.K. are inventors on different patents for γδ T-cell receptor sequences, recognition mechanisms, and isolation strategies. J.K. is the founder and shareholder of Gadeta Founders BV. The remaining authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

