NAT10 promotes hepatocellular carcinoma progression by modulating the ac4C-DDIAS-PI3K-Akt axis
- PMID: 40389420
- PMCID: PMC12089488
- DOI: 10.1038/s41598-025-00707-x
NAT10 promotes hepatocellular carcinoma progression by modulating the ac4C-DDIAS-PI3K-Akt axis
Abstract
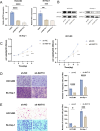
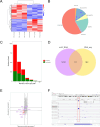
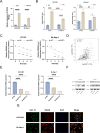
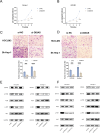
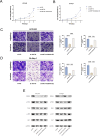
Primary liver cancer (PLC) is a prevalent tumor globally, ranking third in cancer-related mortality. The role of N4-acetylcysteine (ac4C) and N-acetyltransferase 10 (NAT10) in hepatocellular carcinoma (HCC) progression, migration, and invasion requires further elucidation. High NAT10 expression correlated with poor prognosis in HCC patients. Knockdown of NAT10 hindered HCC cell proliferation. AcRIP-seq screening revealed DDIAS as a significant downstream target of NAT10. Decreased NAT10 levels reduced DDIAS mRNA stability, leading to decreased proliferation, migration, and invasion of HCC cells upon DDIAS knockdown. Ectopic expression of DDIAS counteracted the effects of NAT10 knockdown by modulating the PI3K/AKT pathway. NAT10 was found to be elevated in HCC tissues compared to normal tissues, promoting HCC progression and correlating with shorter overall survival in patients. Mechanistically, NAT10 regulated HCC progression through the ac4C-DDIAS-PI3K-AKT axis.
Keywords: Acetylation; DDIAS; Hepatocellular carcinoma; NAT10; PI3K-AKT; ac4C.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

