Intestinal permeability of N-acetylcysteine is driven by gut microbiota-dependent cysteine palmitoylation
- PMID: 40389439
- PMCID: PMC12089494
- DOI: 10.1038/s41467-025-59916-7
Intestinal permeability of N-acetylcysteine is driven by gut microbiota-dependent cysteine palmitoylation
Abstract
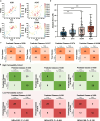
Trillions of intestinal microbiota are essential to the permeability of orally administered drugs. However, identifying microbial-drug interactions remains challenging due to the highly variable composition of intestinal flora among individuals. Using single-pass intestinal perfusion (SPIP) platform, we establish the microbiota-based permeability screening framework involving germ-free (GF) and specific-pathogen-free (SPF) rats to compare in-situ Peff-values and metabolomic profiles of 32 orally administered drugs with disputable classifications of permeability, prior to the verifications of bioorthogonal chemistry and LC-MS/MS. In contrast with SPF controls, N-Acetylcysteine (NAC) exhibits significantly increased permeability in GF rats, which is inversely related to reduced cysteine-3-ketosphinganine by Bacteroides. To further validate these microbiome features, we integrate clinical descriptors from a prospective cohort of 319 participants to optimize a 15-feature eXtreme Gradient Boosting (XGB) model, which reveal that cysteine palmitoylation by intestinal microbiota has significantly affected NAC permeability. By comparison of net reclassification improvement (NRI) index, this machine learning (ML) model of clinical prediction model encompassing intestinal microbial features outperforms other three commercial models in predicting NAC permeability. Here we have developed an intestinal microbiota-based strategy to evaluate uncharacterized NAC permeability, thus accounting for its discordant biopharmaceutics classification.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Amidon, G. L., Lennernas, H., Shah, V. P. & Crison, J. R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res.12, 413–420 (1995). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

