Dental biofilms contain DNase I-resistant Z-DNA and G-quadruplexes but alternative DNase overcomes this resistance
- PMID: 40389511
- PMCID: PMC12089357
- DOI: 10.1038/s41522-025-00694-x
Dental biofilms contain DNase I-resistant Z-DNA and G-quadruplexes but alternative DNase overcomes this resistance
Abstract
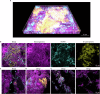
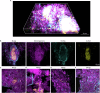
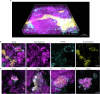
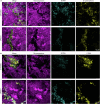
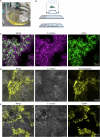
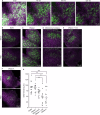
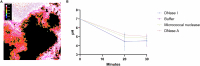
Extracellular DNA (eDNA) in bacterial biofilms can form non-canonical structures like Z-DNA and G-quadruplex (G4), which enhance biofilm resilience by providing protection against mammalian DNases. However, the conformation of eDNA in dental biofilms remains unexplored. Using fluorescence immunolabeling and confocal microscopy, we examined dental biofilms from healthy and caries-active subjects, revealing B-DNA, G4-, and Z-DNA structures surrounding clusters of bacteria, with some structures directly associated with the bacterial cell surface. We demonstrated that these non-canonical DNA structures were resistant to mammalian DNase I. Using a Streptococcus mutans biofilm model, we visualised fluorescently labelled eDNA during enzyme treatment and identified both an experimental nuclease and a DNase I-chloroquine combination capable of removing eDNA that was resistant to DNase I. These findings suggest that G4 and Z-DNA structures represent novel targets for improved enzyme formulations in controlling dental biofilms and potentially other biofilms containing these secondary DNA structures.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: This research was partially funded by Novonesis A/S Denmark. Novonesis A/S Denmark also produced the experimental enzyme used in this study. D.C.S.E., L.G.P., M.K.T., and I.K. were all employees of Novonesis A/S during the time of conducting research and writing. The other authors declare no financial or non-financial competing interests.
Figures

Similar articles
-
Extracellular G-quadruplexes and Z-DNA protect biofilms from DNase I, and G-quadruplexes form a DNAzyme with peroxidase activity.Nucleic Acids Res. 2024 Feb 28;52(4):1575-1590. doi: 10.1093/nar/gkae034. Nucleic Acids Res. 2024. PMID: 38296834 Free PMC article.
-
Extracellular Z-DNA Enhances Cariogenicity of Biofilm.J Dent Res. 2025 Jul;104(7):774-783. doi: 10.1177/00220345251316822. Epub 2025 Mar 12. J Dent Res. 2025. PMID: 40077829
-
High release of Candida albicans eDNA as protection for the scaffolding of polymicrobial biofilm formed with Staphylococcus aureus and Streptococcus mutans against the enzymatic activity of DNase I.Braz J Microbiol. 2024 Dec;55(4):3921-3932. doi: 10.1007/s42770-024-01550-4. Epub 2024 Oct 31. Braz J Microbiol. 2024. PMID: 39480631
-
Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms.Front Cell Infect Microbiol. 2015 Feb 13;5:10. doi: 10.3389/fcimb.2015.00010. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25763359 Free PMC article. Review.
-
Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides.Crit Rev Microbiol. 2021 Sep;47(5):667-677. doi: 10.1080/1040841X.2021.1915959. Epub 2021 May 3. Crit Rev Microbiol. 2021. PMID: 33938347 Review.
Cited by
-
Immune dysfunction during S. aureus biofilm-associated implant infections: opportunities for novel therapeutic strategies.NPJ Biofilms Microbiomes. 2025 Jul 25;11(1):144. doi: 10.1038/s41522-025-00782-y. NPJ Biofilms Microbiomes. 2025. PMID: 40715142 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

