Heterogeneity and efficacy of immunotherapy in multiple cancer: insights from a meta-analysis
- PMID: 40389815
- PMCID: PMC12090696
- DOI: 10.1186/s12575-025-00274-5
Heterogeneity and efficacy of immunotherapy in multiple cancer: insights from a meta-analysis
Abstract
Background: Immunotherapy has been recognized as a significant advancement in cancer treatment by promoting the body's immune system to identify and eliminate cancer cells more effectively. Unlike conventional therapies, immunotherapy can enhance the natural capabilities of human immune system. Chimeric Antigen Receptor T-cell (CAR-T) therapy involves genetical-modified T-cells from patients to better catch and attack cancer cells. Up to date, CAR-T therapy has shown particular promise in treating certain types of leukemia and lymphoma, highlighting the transformative potential of immunotherapy.
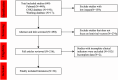
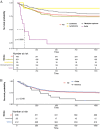
Results: Literature data search using PubMed, CNKI, and Wanfang were searched to collect eligible studies up to January 2025. The primary outcomes of complete response rate (CRR), objective response rate (ORR), dead rate (DR), and other adverse reactions were evaluated. Secondary outcomes (CRR, ORR, and DR) of subgroup analysis from different cancer types, origins, and outcomes for survival rate were analyzed for our final results. A total of 649 studies were initially identified through database searching. After removing duplicates and non-clinical cancer studies, 32 eligible studies were included in this work. The pooled data included 819 patients for objective response rate (ORR), 843 patients for complete response rate (CRR), and 868 patients for dead event. In the included studies, 24 reported ORR data, revealing an objective response rate of 84.86% (695/819) with little heterogeneity (OR: 0.87, 95% CI 0.80-0.91, P = < 0.01, I2 = 61%); 24 studies showed a CRR of 65.30% (491/843) with significant heterogeneity (OR: 0.58, 95% CI: 0.43-0.72, P < 0.01, I2 = 84%); 27 studies showed a mortality rate of 23.73% (206/868) with significant heterogeneity (OR: 0.19, 95% CI: 0.11-0.32, P < 0.01, I2 = 77%). Subgroup analysis based on cancer type revealed that ORR was higher in multiple myeloma (86.77%, 400/461) compared with leukemia (84.92%, 259/305) and lymphoma (67.92%, 36/53). In parallel, heterogeneity observed based on case origins suggested that Chinese cases showed significantly higher ORR, CRR, and survival rates compared with American ones.
Conclusions: This meta-analysis provides valuable insights into the potential of immunotherapy, particularly CAR-T, in cancer treatment. Findings showed the different efficacy and safety of immunotherapy in treating multiple cancers, with various objective response rates. Continued studies from more trials with different populations are needed to optimize their efficacy in further cancer treatment and precision medicine.
Keywords: Clinical outcomes; Different population; Hematological malignancies; Response rate.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This article does not cover animal or human testing. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Application of CAR-T cell therapy in B-cell lymphoma: a meta-analysis of randomized controlled trials.Clin Transl Oncol. 2025 Jun;27(6):2700-2709. doi: 10.1007/s12094-024-03774-0. Epub 2024 Nov 8. Clin Transl Oncol. 2025. PMID: 39514165
-
Efficacy and Safety of CAR-Modified T Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma: A Meta-Analysis of Prospective Clinical Trials.Front Pharmacol. 2020 Dec 3;11:544754. doi: 10.3389/fphar.2020.544754. eCollection 2020. Front Pharmacol. 2020. PMID: 33343342 Free PMC article. Review.
-
Comprehensive meta-analysis of anti-BCMA chimeric antigen receptor T-cell therapy in relapsed or refractory multiple myeloma.Ann Med. 2021 Dec;53(1):1547-1559. doi: 10.1080/07853890.2021.1970218. Ann Med. 2021. PMID: 34459681 Free PMC article.
-
Effectiveness and safety of anti-BCMA chimeric antigen receptor T-cell treatment in relapsed/refractory multiple myeloma: a comprehensive review and meta-analysis of prospective clinical trials.Front Pharmacol. 2023 Jun 20;14:1149138. doi: 10.3389/fphar.2023.1149138. eCollection 2023. Front Pharmacol. 2023. PMID: 37408760 Free PMC article.
-
Efficacy and safety of chimeric antigen receptor T cells targeting BCMA and GPRC5D in relapsed or refractory multiple myeloma.Front Immunol. 2024 Dec 23;15:1466443. doi: 10.3389/fimmu.2024.1466443. eCollection 2024. Front Immunol. 2024. PMID: 39763668 Free PMC article.
References
-
- Bao Y. Study on the efficacy and safety of BCMA-targeted CAR-T in the treatment of relapsed/refractory multiple myeloma. Beijing: Tongfang Knowledge Network (Beijing) Technology Co., Ltd.; 2023. 10.27438/d.cnki.gyadu.2022.000460.
-
- Blumenschein GR, Devarakonda S, Johnson M, Moreno V, Gainor J, Edelman MJ, Heymach JV, Govindan R, Bachier C, Doger DSB, Matthew JF, Olszanski AJ, Vincent KL, Hyland N, Navenot JM, Fayngerts S, Wolchinsky Z, Broad R, Batrakou D, Pentony MM, Sanderson JP, Gerry A, Marks D, Bai J, Holdich T, Norry E, Fracasso PM. Phase I clinical trial evaluating the safety and efficacy of ADP-A2M10 SPEAR T cells in patients with MAGEA10+ advanced non-small cell lung cancer. J ImmunoTherap Cancer. 2022;10(1):e003581. 10.1136/jitc-2021-003581. - PMC - PubMed
-
- Brudno JN, Natrakul DA, Karrs J, Patel N, Maass-Moreno R, Ahlman MA, Mikkilineni L, Mann J, Stroncek DF, Highfill SL, Fromm GC, Patel R, Pittaluga S, Kochenderfer JN. Transient responses and significant toxicities of anti-CD30 CAR T cells for CD30+ lymphomas: results of a phase 1 trial. Blood Adv. 2024;8(3):802–14. 10.1182/bloodadvances.2023011470. - PMC - PubMed
-
- Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol. 2009;62(12):1253-1260 e1254. 10.1016/j.jclinepi.2008.12.010. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

