GIANT: a prospective, multicenter, real-world study on the effectiveness, safety, and tolerability of atogepant in migraine patients with multiple therapeutic failures
- PMID: 40389834
- PMCID: PMC12090485
- DOI: 10.1186/s10194-025-02068-2
GIANT: a prospective, multicenter, real-world study on the effectiveness, safety, and tolerability of atogepant in migraine patients with multiple therapeutic failures
Abstract
Background: Atogepant, the first oral CGRP receptor antagonist approved for migraine prevention, has demonstrated efficacy and safety in randomized clinical trials (RCT). However, prospective real-world data are lacking.
Objective: To explore the effectiveness, safety, and tolerability of atogepant 60 mg at week 12 in patients with high-frequency episodic (HFEM: 8-14 days/month) or chronic migraine (CM) with multiple therapeutic failures.
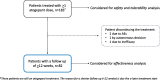
Methods: This ongoing, multicenter (n = 16), prospective real-world study included consecutive adults with HFEM or CM who had failed ≥3 prior preventive treatments, according to AIFA criteria. Participants received atogepant 60 mg daily, with treatment planned for up to 12 months.
Primary endpoint: change from baseline to week 12 in monthly migraine days (MMD) for HFEM and monthly headache days (MHD) for CM. Secondary endpoints: changes in monthly analgesic intake (MAI), pain intensity (NRS), disability (HIT-6, MIDAS), interictal burden (MIBS-4), treatment satisfaction (PGIC), responder rates (≥ 50%, ≥ 75%, 100%), and changes in migraine frequency during the first treatment week compared to the last pre-treatment week. Adverse events were monitored throughout.
Results: A total of 183 patients were enrolled and 82 completed ≥ 12 weeks of follow-up. Of these, 41.5% had previously failed anti-CGRP mAbs. At week 12, significant reductions (p < 0.001) were observed in MMD (-6.0) and MHD (-11.2). Secondary outcomes also improved significantly (p < 0.001): MAI (-10.9), NRS (-2.7), HIT-6 (-13.2), MIDAS (-61.1), and MIBS-4 (-5.4). Responder rates were 65.9% (≥ 50%), 36.6% (≥ 75%), and 6.1% (100%). PGIC documented high satisfaction (much/very much improved: 70.7%). A significant decrease (p < 0.001) in migraine frequency was already evident by week 1 (overall: - 2.5 days, HFEM: - 1.5, CM: - 3.1). In the mAb-failure subgroup, ≥ 50% and ≥ 75% response rates were 52.9% and 23.5%, with significant improvements in all primary and secondary endpoints (p < 0.001). Adverse events occurred in 5.5% of patients, and 1.6% discontinued treatment.
Conclusion: The GIANT study provides real-world evidence of atogepant's effectiveness, safety, and tolerability in patients with HFEM and CM with multiple therapeutic failures and comorbidities. It extends RCT data by showing rapid onset of action, meaningful reductions in pain intensity and interictal disability, high patient satisfaction, and effectiveness even in patients with anti-CGRP mAb failures.
Keywords: Atogepant; CGRP; Disability; Migraine; Real-world; Treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All patients provided written informed consent prior to participation. The study was conducted in accordance with the Declaration of Helsinki, approved by the Lazio 5 Institutional Review Board (approval number 0003898), and mutually recognized by the ethics committees of all participating centers. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P (2024) CGRP monoclonal antibodies and CGRP receptor antagonists (Gepants) in migraine prevention. Handb Clin Neurol 199:107–124. 10.1016/B978-0-12-823357-3.00024-0 - PubMed
-
- Li D, Abreu J, Tepper SJ (2023) A brief review of gepants. Curr Pain Headache Rep 27(9):479–488. 10.1007/s11916-023-01142-1 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

