Predictive value of growth hormone and insulin-like growth factor-1 axis for gestational diabetes mellitus: a prospective cohort study
- PMID: 40389967
- PMCID: PMC12087125
- DOI: 10.1186/s12902-025-01953-w
Predictive value of growth hormone and insulin-like growth factor-1 axis for gestational diabetes mellitus: a prospective cohort study
Abstract
Objective: This study aimed to explore the role of growth hormone/insulin-like growth factor-1 risk factor axis in gestational diabetes mellitus, as well as to rank independently risk factors.
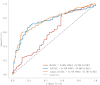
Methods: This was a prospective cohort study conducted between April 2019 and April 2022. The baseline data and serum samples were collected and analyzed from 241 pregnant women during the second trimester. Logistic regression and restricted cubic spline analyses were conducted to assess the relationship between GH and IGF-1 correlated with risk of GDM. Back-propagation artificial neural network (BPNN) and Receiver operating characteristic (ROC) curve analysis were performed to identify the predictive ability of the GH/IGF-1 axis for GDM.
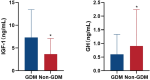
Results: The present study found that the higher serum levels of IGF-1 and the lower serum levels of GH in pregnant women were significantly correlated with risk of GDM. GH and IGF-1 were different in both case and control groups(P < 0.05). BPNN analysis identified IGF-1 as accounting for the highest proportion in the ranking of GDM risk prediction weights (up to 25.4%). Furthermore, the area under ROC curve (AUC) value of the GH and IGF-1 combinations reached 0.770 (95%CI:0.707, 0.83).
Conclusions: GH (growth hormone) and IGF-1 (insulin-like growth factor 1) are intricately linked to the development of gestational diabetes mellitus (GDM). Disruptions in the GH/IGF-1 axis can trigger insulin resistance, thereby elevating the risk of GDM.
Trial registration: Current Controlled Trials: ChiCTR2000028811. Registration Date:20,200,104.
Keywords: Gestational diabetes mellitus; Growth hormone; Insulin resistance; Insulin-like growth factor-I risk factor; Serum.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Clinical Trial Ethics Committee of the Third Afliated Hospital of Zhengzhou University, and the study had been registered with the Chinese Clinical Trial Registry (ChiCTR2000028811). The study adhered to the Declaration of Helsinki. Consent to participate: Informed consent was provided by all participants before they were recruited for the study, and data were analyzed anonymously. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

