Exosomes derived from M2 macrophages regulate airway inflammation by modulating epithelial cell proliferation and apoptosis
- PMID: 40390011
- PMCID: PMC12090695
- DOI: 10.1186/s12950-025-00444-y
Exosomes derived from M2 macrophages regulate airway inflammation by modulating epithelial cell proliferation and apoptosis
Abstract
Background: Asthma is a chronic inflammatory disease characterized by airway remodeling and immune dysregulation. This study aimed to explore the mechanisms by which M2 macrophage-derived exosomes (M2Φ-Exos) regulate airway inflammation in asthma by modulating epithelial cell proliferation and apoptosis.
Methods: M2Φ-Exos were extracted and characterized by morphology, size, and marker protein expression. In vitro, the effects of M2Φ-Exos on House Dust Mites (HDM)-stimulated mouse lung epithelial cells (MLE-12s) were evaluated using western blotting to analyze Proliferating Cell Nuclear Antigen (PCNA), B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), and cleaved caspase-3 expression. In vivo, M2Φ-Exos were administered to HDM-induced asthmatic mice to assess their impact on airway inflammation, epithelial remodeling, and proliferation-apoptosis balance using immunohistochemistry, immunofluorescence, and western blotting. Cytokine levels in lung tissue and bronchoalveolar lavage fluid (BALF) were measured by qRT-PCR and ELISA.
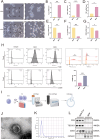
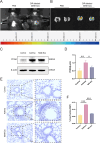
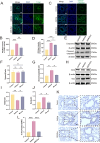
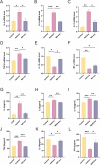
Results: M2Φ-Exos displayed typical cup-shaped morphology, an average diameter of 115.5 nm, and expressed marker proteins CD9, TSG101, and CD63. MLE-12 cells internalized M2Φ-Exos, leading to reduced abnormal proliferation and apoptosis in HDM-stimulated cells. In asthmatic mice, M2Φ-Exos alleviated airway inflammation and epithelial thickening while reducing PCNA, cleaved caspase-3, and Bax levels and increasing Bcl-2 expression. M2Φ-Exos suppressed pro-inflammatory cytokines (IL-4, IL-5, IL-13) and Transforming growth factor (TGF)-β, while enhancing anti-inflammatory cytokine IFN-γ and IL-10.
Conclusion: These findings demonstrate that M2Φ-Exos regulate the imbalance in epithelial proliferation and apoptosis in asthma, reducing inflammation and mitigating tissue remodeling, and provide new insights into potential therapeutic strategies for asthma management.
Keywords: Apoptosis; Asthma; Exosome; M2 macrophage; Proliferation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experimental procedures were reviewed and approved by the Ethics Committee of the Children’s Hospital of Chongqing Medical University (Approval Numbers: 0918001). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
M2 macrophage-derived exosomes reverse TGF-β1-induced epithelial mesenchymal transformation in BEAS-2B cells via the TGF-βRI/Smad2/3 signaling pathway.Eur J Med Res. 2025 Apr 11;30(1):271. doi: 10.1186/s40001-025-02516-4. Eur J Med Res. 2025. PMID: 40211426 Free PMC article.
-
Scorpion and centipede alleviates severe asthma through M2 macrophage-derived exosomal miR-30b-5p.Aging (Albany NY). 2022 May 2;14(9):3921-3940. doi: 10.18632/aging.204053. Epub 2022 May 2. Aging (Albany NY). 2022. PMID: 35500231 Free PMC article.
-
MicroRNA-370 carried by M2 macrophage-derived exosomes alleviates asthma progression through inhibiting the FGF1/MAPK/STAT1 axis.Int J Biol Sci. 2021 Apr 23;17(7):1795-1807. doi: 10.7150/ijbs.59715. eCollection 2021. Int J Biol Sci. 2021. PMID: 33994863 Free PMC article.
-
Adipose Stem Cell-Derived Exosomes Ameliorate Chronic Rotator Cuff Tendinopathy by Regulating Macrophage Polarization: From a Mouse Model to a Study in Human Tissue.Am J Sports Med. 2021 Jul;49(9):2321-2331. doi: 10.1177/03635465211020010. Am J Sports Med. 2021. PMID: 34259608
-
[Effects of exosomes from human adipose-derived mesenchymal stem cells on inflammatory response of mouse RAW264.7 cells and wound healing of full-thickness skin defects in mice].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022 Mar 20;38(3):215-226. doi: 10.3760/cma.j.cn501120-20201116-00477. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022. PMID: 35325966 Free PMC article. Chinese.
References
-
- Shipp CL, Gergen PJ, Gern JE, Matsui EC, Guilbert TW. Asthma management in children. J Allergy Clin Immunol Pract. 2023;11:9–18. - PubMed
-
- Zhu C, Sun Y, Zhao Y, Hou J, Zhang Q, Wang P. Associations between children’s asthma and allergic symptoms and phthalates in dust in metropolitan Tianjin, China. Chemosphere. 2022;302:134786. - PubMed
-
- Shin YH, Hwang J, Kwon R, Lee SW, Kim MS, Shin JI, Yon DK, GBD 2019 Allergic Disorders Collaborators. Global, regional, and National burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: a systematic analysis for the global burden of disease study 2019. Allergy. 2023;78:2232–54. - PMC - PubMed
Grants and funding
- KJQN202200411/Science and Technology Research Program of Chongqing Municipal Education Commission
- KJQN202200419/Science and Technology Research Program of Chongqing Municipal Education Commission
- CSTC2020jcyj-msxmX0615/Scientific Natural Science Foundation of Chongqing
- CSTB2022NSCQ-MSX0930/Scientific Natural Science Foundation of Chongqing
- 2022MD713713/Project of China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

