Bayesian trial of adalimumab versus secukinumab for children with juvenile idiopathic arthritis associated uveitis or chronic anterior uveitis
- PMID: 40390069
- PMCID: PMC12090588
- DOI: 10.1186/s12969-025-01107-1
Bayesian trial of adalimumab versus secukinumab for children with juvenile idiopathic arthritis associated uveitis or chronic anterior uveitis
Abstract
Background: Juvenile idiopathic arthritis (JIA)-associated uveitis and chronic anterior uveitis in children may result in permanent sight loss. Currently, the only licensed and approved treatment for JIA-uveitis is adalimumab. However, even in patients where adalimumab may be initially effective, therapeutic response may subside for example, due to neutralising drug antibodies. Further treatment options are necessary to prevent sight loss in children with uveitis. Interleukin 17 is elevated in uveitis. Inhibition of interleukin 17 ameliorates inflammation in mouse models of uveitis. Secukinumab, an antibody which neutralizes interleukin 17 A, has been shown to be partially effective in adult uveitis. The objective of the Bayesian consensus meeting was to quantify prior expert opinion about the potential utility of secukinumab in treatment of uveitis in JIA.
Methods: Nine international experts in paediatric rheumatology, paediatric ophthalmology and/or paediatric uveitis took part in a structured Bayesian prior elicitation meeting.
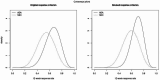
Results: The final consensus was that adalimumab is expected to yield a higher response rate than secukinumab (mean 0.67 vs. 0.55). The uncertainty in the response rate on secukinumab is somewhat larger than for adalimumab. The equivalent sample size for the prior distribution of adalimumab is 15.7 and 13.1 for secukinumab. The decisions based on the combined evidence would still be driven by the trial data, yet substantial enhancement of the power of the study can be expected by adding information from the equivalent of almost 30 patients.
Conclusions: The Bayesian analysis adds substantial enhancement of the power of the study and supports a head-to-head trial of adalimumab and secukinumab for JIA-associated uveitis and chronic anterior uveitis.
Trial registration: ISRCTN 12,427,150 Registration date 14/02/2023. EudraCT 2022-003068-26 Registration date 07/09/2022.
Keywords: Bayesian prior; Biologics; Clinical trial; Juvenile idiopathic arthritis; Uveitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: ADD is Co-Director of the National Institute of Health Research Moorfields Biomedical Research Centre, Moorfields and UCL-Institute of Ophthalmology. TJ and DSR also received funding from UK Medical Research Council (MC_UU_00002/14 and MC_UU_00040/03).
Figures

Similar articles
-
A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE Trial).Trials. 2014 Jan 9;15:14. doi: 10.1186/1745-6215-15-14. Trials. 2014. PMID: 24405833 Free PMC article. Clinical Trial.
-
Clinical effectiveness and safety of baricitinib for the treatment of juvenile idiopathic arthritis-associated uveitis or chronic anterior antinuclear antibody-positive uveitis: study protocol for an open-label, adalimumab active-controlled phase 3 clinical trial (JUVE-BRIGHT).Trials. 2021 Oct 9;22(1):689. doi: 10.1186/s13063-021-05651-5. Trials. 2021. PMID: 34627340 Free PMC article.
-
Adalimumab for juvenile idiopathic arthritis-associated uveitis.Graefes Arch Clin Exp Ophthalmol. 2013 Jun;251(6):1601-6. doi: 10.1007/s00417-013-2275-x. Epub 2013 Mar 1. Graefes Arch Clin Exp Ophthalmol. 2013. PMID: 23446556
-
Juvenile idiopathic arthritis-associated uveitis.Best Pract Res Clin Rheumatol. 2017 Aug;31(4):517-534. doi: 10.1016/j.berh.2018.01.002. Epub 2018 Feb 26. Best Pract Res Clin Rheumatol. 2017. PMID: 29773271 Review.
-
Management of JIA associated uveitis.Best Pract Res Clin Rheumatol. 2024 Sep;38(3):101979. doi: 10.1016/j.berh.2024.101979. Epub 2024 Jul 23. Best Pract Res Clin Rheumatol. 2024. PMID: 39048481 Review.
References
-
- Kautiained H, Karma A, Aho K. Occurrence of uveitis in recently diagnosed juvenile chronic arthritis: a prospective study. Kotaniemi K. Volume 108. Ophthalmology; 2001. 2071-5, s.l. - PubMed
-
- Prevalence., risk factors, and outcome of uveitis in juvenile idiopathic arthritis: a long-term followup. Saurenmann RK, Levin AV, Feldman BM, Rose JB, Laxer RM, Schneider R, Silverman ED. 56:647– 57, s.l.: Arthritis Rheum, 2007. - PubMed
-
- Denove GN, Yu CS. Chronic anterior uveitis in children: clinical characteristics and. Holland. Am J Ophthalmol. F. 2009;147:667–78. s.l. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

