RNA polymerase I is essential for driving the formation of 3D genome in early embryonic development in mouse, but not in human
- PMID: 40390095
- PMCID: PMC12087037
- DOI: 10.1186/s13073-025-01476-y
RNA polymerase I is essential for driving the formation of 3D genome in early embryonic development in mouse, but not in human
Abstract
Background: Three-dimensional (3D) chromatin architecture undergoes dynamic reorganization during mammalian gametogenesis and early embryogenesis. While mouse studies have shown species-specific patterns as well as mechanisms underlying de novo organization, these remain poorly characterized in humans. Although RNA polymerases II and III have been shown to regulate chromatin structure, the potential role of RNA polymerase I (Pol I), which drives ribosomal RNA production, in shaping 3D genome organization during these developmental transitions has not been investigated.
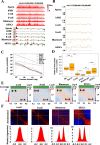
Methods: We employed a modified low-input in situ Hi-C approach to systematically compare 3D genome architecture dynamics from gametogenesis through early embryogenesis in human and mouse. Complementary Smart-seq2 for low-input transcriptomics, CUT&Tag for Pol I profiling, and Pol I functional inhibition assays were performed to elucidate the mechanisms governing chromatin organization.
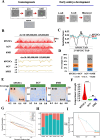
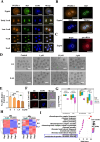
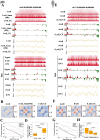
Results: Our study revealed an extensive reorganization of the 3D genome from human oogenesis to early embryogenesis, displaying significant differences with the mouse, including dramatically attenuated topologically associating domains (TADs) at germinal vesicle (GV) stage oocytes. The 3D genome reconstruction timing is a fundamental difference between species. In human, reconstruction initiates at the 4-cell stage embryo in human, while in mouse, it commences at the 2-cell stage embryo. We discovered that Pol I is crucial for establishing the chromatin structures during mouse embryogenesis, but not in human embryos. Intriguingly, the absence of Pol I transcription weakens TAD structure in mouse female germline stem cells, whereas it fortifies it in human counterparts.
Conclusions: These observed interspecies distinctions in chromatin organization dynamics provide novel insights into the evolutionary divergence of chromatin architecture regulation during early mammalian development. Our findings provide mechanistic insights into species-specific chromatin organization during germ cell and embryonic development and have potential implications for fertility preservation and birth defect prevention.
Keywords: Chromatin structure; Early embryonic development; Polymerase I; Stem cell.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the institutional review boards of The Affiliated Ren Ji Hospital of Shanghai Jiao Tong University (2019122701), Affiliated Tongji Hospital of Tongji University (2020-KYSB- 048), Shanghai First Maternity, and Infant Hospital (KS21280), The First Affiliated Hospital of Anhui Medical University (2020H026), and Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (2020–866 - 75–01). The study was conducted in accordance with the measures of the People’s Republic of China on the administration of human-assisted reproductive technology, the ethical principles of human-assisted reproductive technology, and the Helsinki Declaration. All participants provided written informed consent to participate in the study. All animal experiments were conducted in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and relevant Chinese laws and regulations, and were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University (Spatiotemporal dynamics and regulatory mechanisms of gametogenesis and embryonic development in mammals, A2019118). Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Super RNA Pol II domains enhance minor ZGA through 3D interaction to ensure the integrity of major transcriptional waves in late-ZGA mammals.Cell Genom. 2025 Jun 11;5(6):100856. doi: 10.1016/j.xgen.2025.100856. Epub 2025 May 1. Cell Genom. 2025. PMID: 40315839 Free PMC article.
-
Three-dimensional genome structure shapes the recombination landscape of chromatin features during female germline stem cell development.Clin Transl Med. 2022 Jun;12(6):e927. doi: 10.1002/ctm2.927. Clin Transl Med. 2022. PMID: 35730671 Free PMC article.
-
Parental-to-embryo switch of chromosome organization in early embryogenesis.Nature. 2020 Apr;580(7801):142-146. doi: 10.1038/s41586-020-2125-z. Epub 2020 Mar 25. Nature. 2020. PMID: 32238933
-
The Dynamic 3D Genome in Gametogenesis and Early Embryonic Development.Cells. 2019 Jul 29;8(8):788. doi: 10.3390/cells8080788. Cells. 2019. PMID: 31362461 Free PMC article. Review.
-
The Birth of the 3D Genome during Early Embryonic Development.Trends Genet. 2018 Dec;34(12):903-914. doi: 10.1016/j.tig.2018.09.002. Epub 2018 Oct 3. Trends Genet. 2018. PMID: 30292539 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

