Medication use in the neonatal intensive care unit in a tertiary hospital in China from 2020 to 2023
- PMID: 40390107
- PMCID: PMC12090553
- DOI: 10.1186/s13052-025-02003-w
Medication use in the neonatal intensive care unit in a tertiary hospital in China from 2020 to 2023
Abstract
Background: The use of medication in the neonatal intensive care unit (NICU) is a complex field that requires special attention, as neonatal patients may have different sensitivities and responses to drugs than adults and older children. The administration of medication in the NICU must consider various factors, including the dosage of the medication, the route of administration, monitoring, and potential drug interactions.
Methods: In this study, we conducted a retrospective analysis of medication use in the neonatal intensive care unit of 122 preterm infants treated in our hospital from 2020 to 2023.
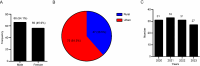
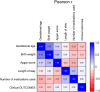
Results: Correlation analysis revealed that among perinatal clinical characteristics, birth weight was moderately positively correlated with gestational age, with a correlation coefficient greater than that of birth weight with the Apgar score. The top 3 medication types in the NICU were "vitamins, nutritional drugs, enzyme preparations and drugs that regulate water, electrolytes, and acid-base balance", "blood hematopoietic system medications", and "antimicrobial medications". From 2020 to 2023, the most commonly used drugs in the NICU were vitamin AD (vitamin A and vitamin D) drops and calcium gluconate injections. In addition, we demonstrated that the most commonly prescribed off-label drugs were vitamins, water and electrolyte balance nutrition drugs, and blood circulation system drugs.
Conclusions: Our retrospective study will not only help identify and evaluate interventions to reduce medication errors but also aid healthcare systems and providers in understanding, implementing, and enhancing these interventions to improve the safety and quality of care for newborns. Nonetheless, further research is needed to assess the relative cost-effectiveness of various medication safety interventions to facilitate their adoption and implementation in the decision-making process.
Keywords: Clinical pharmacology; Neonatal intensive care unit; Off-label drug; Pharmacotherapy; Trends over time.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of the Seventh Medical Center of PLA General Hospital (S2024-046-01) and consisted of the Declaration of Helsinki. The studies were conducted in accordance with local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because of the retrospective design of the study. Consent for publication: Not applicable. Competing interests: On behalf of all the authors, the corresponding author states that there are no conflicts of interest.
Figures
References
-
- Sharma A, Sharma N, Sharma A. Soft tissue therapy in managing neonatal procedural pain: a systematic review. J Neonatal Nurs. 2023;29(6):815–. 10.1016/j.jnn.2023.02.011 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

