The progress and prospects of targeting the adenosine pathway in cancer immunotherapy
- PMID: 40390144
- PMCID: PMC12090549
- DOI: 10.1186/s40364-025-00784-0
The progress and prospects of targeting the adenosine pathway in cancer immunotherapy
Abstract
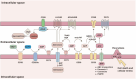
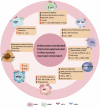
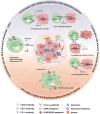
Despite the notable success of cancer immunotherapy, its effectiveness is often limited in a significant proportion of patients, highlighting the need to explore alternative tumor immune evasion mechanisms. Adenosine, a key metabolite accumulating in hypoxic tumor regions, has emerged as a promising target in oncology. Inhibiting the adenosinergic pathway not only inhibits tumor progression but also holds potential to enhance immunotherapy outcomes. Multiple therapeutic strategies targeting this pathway are being explored, ranging from preclinical studies to clinical trials. This review examines the complex interactions between adenosine, its receptors, and the tumor microenvironment, proposing strategies to target the adenosinergic axis to boost anti-tumor immunity. It also evaluates early clinical data on pharmacological inhibitors of the adenosinergic pathway and discusses future directions for improving clinical responses.
Keywords: Adenosine; Adenosine receptors; CD39; CD73; Cancer immunotherapy; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors approved the final version of the manuscript. Competing interests: The authors declare no competing interests.
Figures

References
Publication types
Grants and funding
- No. 2023M743967/fellowship of China Postdoctoral Science Foundation
- No. 2024JJ6684/Natural Science Foundation of Hunan Province
- No. 2023Q20/Youth Science Foundation of Xiangya Hospital
- No. kq2403025/Natural Science Foundation of Changsha
- No. 2022YFC2504700/National Key Research and Development Program of China
- No. 82130090/Key Program of National Natural Science Foundation of China
- No. 2023JJ10096/Science Fund for Distinguished Young Scholars of Hunan Province
- No. 2022RC1215/Science and Technology Innovation Program of Hunan Province
- U22A20329/key Program of National Natural Science Foundation of China
- 2023QYJC004/Central South University Research Programme of Advanced Interdisciplinary Studies
- No. 2023SK2095/the Scientific Research Program of FuRong Laboratory
LinkOut - more resources
Full Text Sources
Research Materials

