Mechanisms of alcohol influence on fear conditioning: A computational model
- PMID: 40390190
- PMCID: PMC12173769
- DOI: 10.1111/acer.70071
Mechanisms of alcohol influence on fear conditioning: A computational model
Abstract
Background: A connection between stress-related illnesses and alcohol use disorders is extensively documented. Fear conditioning is a standard procedure used to study stress learning and links it to the activation of amygdala circuitry. However, the connection between the changes in amygdala circuitry and function induced by alcohol and fear conditioning is not well established.
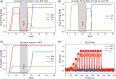
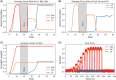
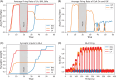
Methods: We introduce a computational model to test the mechanistic relationship between amygdala functional and circuit adaptations during fear conditioning and the impact of acute vs. repeated alcohol exposure. Using firing rate formalism, the model generates electrophysiological and behavioral responses in fear conditioning protocols via plasticity of amygdala inputs. The influence of alcohol is modeled by accounting for known modulation of connections within amygdala circuits, which consequently affect plasticity. Thus, the model connects the electrophysiological and behavioral experiments. We hypothesize that alterations within amygdala circuitry produced by alcohol cause abnormal plasticity of amygdala inputs such that fear extinction is slower to achieve and less robust.
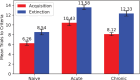
Results: In accordance with prior experimental results, both acute and prior repeated alcohol decrease the speed and robustness of fear extinction in our simulations. The model predicts that, first, the delay in fear extinction caused by alcohol is mostly induced by greater activation of the basolateral amygdala (BLA) after fear acquisition due to alcohol-induced modulation of synaptic weights. Second, both acute and prior repeated alcohol shift the amygdala network away from the robust extinction regime by inhibiting activity in the central amygdala (CeA). Third, our model predicts that fear memories formed during acute or after chronic alcohol are more connected to the context.
Conclusions: The model suggests how circuit changes induced by alcohol may affect fear behaviors and provides a framework for investigating the involvement of multiple neuromodulators in this neuroadaptive process.
Keywords: amygdala circuit; computational model; electrophysiological activity; fear acquisition; fear extinction.
© 2025 The Author(s). Alcohol, Clinical and Experimental Research published by Wiley Periodicals LLC on behalf of Research Society on Alcohol.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Update of
-
Mechanisms of alcohol influence on fear conditioning: a computational model.bioRxiv [Preprint]. 2024 Jan 1:2023.12.30.573310. doi: 10.1101/2023.12.30.573310. bioRxiv. 2024. Update in: Alcohol Clin Exp Res (Hoboken). 2025 Jun;49(6):1233-1247. doi: 10.1111/acer.70071. PMID: 38260700 Free PMC article. Updated. Preprint.
References
-
- Bertotto, M.E. , Bustos, S.G. , Molina, V.A. & Martijena, I.D. (2006) Influence of ethanol withdrawal on fear memory: effect of D‐cycloserine. Neuroscience, 142, 979–990. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

