Restoring Prostacyclin/PGI2-PTGIR signaling alleviates intestinal fibrosis in Crohn's disease via fibroblast-specific YAP/TAZ inhibition
- PMID: 40390655
- PMCID: PMC12138779
- DOI: 10.1093/ecco-jcc/jjaf084
Restoring Prostacyclin/PGI2-PTGIR signaling alleviates intestinal fibrosis in Crohn's disease via fibroblast-specific YAP/TAZ inhibition
Erratum in
-
Correction to: Restoring Prostacyclin/PGI2-PTGIR signaling alleviates intestinal fibrosis in Crohn's disease via fibroblast-specific YAP/TAZ inhibition.J Crohns Colitis. 2025 Dec 23;19(12):jjaf142. doi: 10.1093/ecco-jcc/jjaf142. J Crohns Colitis. 2025. PMID: 41452461 Free PMC article. No abstract available.
Abstract
Background and aims: Intestinal obstruction caused by fibrosis is a common and serious complication of Crohn's disease (CD). Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motifs (TAZ), the transcriptional effectors of the Hippo signaling pathway, have emerged as key drivers of intestinal fibrosis. Systematic inhibition of YAP/TAZ failed to combat fibrotic progression, probably due to the vital role of epithelial YAP/TAZ in intestinal homeostasis.
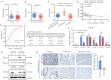
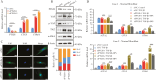
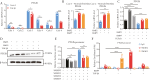
Methods: Enzyme-Linked Immunosorbent Assay (ELISA) and immunohistochemical staining were used to detect serum Prostaglandin I2 (PGI2) levels and PGI2 Receptor (PTGIR) in clinical samples derived from CD patients. Dual luciferase reporter and Cut & Run assays were performed to explore the transcriptional regulatory mechanisms of PTGIR and PGI2 synthase (PTGIS) by tumor necrosis factor α (TNF-α) and transforming growth factor-beta (TGF-β), respectively. Primary intestinal fibroblasts and a chronic colitis model were used for assessing the efficacy of a PTGIR agonist in combating fibrosis.
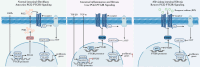
Results: The Gαs-coupled PTGIR is expressed in intestinal fibroblasts but is barely expressed in intestinal epithelial cells. PTGIR transcription is directly activated by p65 in fibroblasts upon TNF-α stimulation. Importantly, PTGIS is transcriptionally suppressed by TGF-β, leading to the loss of endogenous antifibrotic PGI2-PTGIR signaling. Serum PGI2 levels are decreased in CD patients with stenosis and are negatively correlated with disease duration. The PTGIR agonist inhibited the profibrotic function of YAP/TAZ in intestinal fibroblasts in vitro and reversed intestinal fibrosis in vivo.
Conclusions: The antifibrotic effects of PGI2-PTGIR signaling are impaired in CD. Restoring PGI2-PTGIR signaling is a pharmacologically tractable and cell-selective approach to targeting YAP/TAZ via PTGIR, which reverses intestinal fibrosis.
Keywords: Crohn’s disease; intestinal fibrosis; prostacyclin.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
A patent application about predicting intestinal fibrosis via PGI2 detection has been filed.
Figures




References
-
- D’Haens G, Rieder F, Feagan BG, et al. ; International Organization for Inflammatory Bowel Disease Fibrosis Working Group. Challenges in the pathophysiology, diagnosis, and management of intestinal fibrosis in inflammatory bowel disease. Gastroenterology. 2022;162:26–31. https://doi.org/ 10.1053/j.gastro.2019.05.072 - DOI - PMC - PubMed
-
- Wang J, Lin S, Brown JM, van Wagoner D, Fiocchi C, Rieder F.. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev. 2021;302:211–227. https://doi.org/ 10.1111/imr.12974 - DOI - PMC - PubMed
-
- Adamina M, Bonovas S, Raine T, et al. ECCO guidelines on therapeutics in crohn’s disease: surgical treatment. J Crohns Colitis. 2020;14:155–168. https://doi.org/ 10.1093/ecco-jcc/jjz187 - DOI - PubMed
-
- Chan WPW, Mourad F, Leong RW.. Crohn’s disease associated strictures. J Gastroenterol Hepatol. 2018;33:998–1008. https://doi.org/ 10.1111/jgh.14119 - DOI - PubMed
-
- Lin XX, Qiu Y, Zhuang XJ, et al. Intestinal stricture in Crohn’s disease: a 2020 update. J Dig Dis. 2021;22:390–398. https://doi.org/ 10.1111/1751-2980.13022 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

