Simulations-Based Least Required Sample Size and Power in Clinical Trials with Time-to-Event endpoint and Variable Hazard
- PMID: 40390836
- PMCID: PMC12087582
Simulations-Based Least Required Sample Size and Power in Clinical Trials with Time-to-Event endpoint and Variable Hazard
Abstract
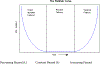
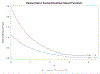
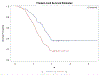
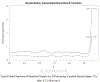
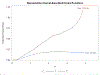
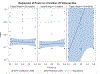
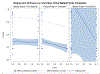
Two of the pivotal design parameters for planning clinical trials with time-to-event outcome(s) are sample size and power. Attention needs to be placed on the hazard function (which characterizes the rate at which events occur and can be constant, decreasing, and/or increasing in time). This work employs simulation(s) of real scenarios of randomized studies to generate time-to-event variables with specific hazard characterization, obeying the Weibull function which accommodates variable hazard situations. Our aim is to determine the least required sample size and power values, based on simulating two independent samples of Weibull distributed responses, differing by various postulated hazard patterns (constant, decreasing, or increasing in time), different scale parameter values, and follow-up periods.
Keywords: Hazard; Simulation; Statistical Power; Time-to-event; Weibull.
Figures
References
-
- Chow S-C, Shao J, Wang H, Lokhnygina Y. Sample size calculations in clinical research, 3rd edition. Chapman & Hall/CRC Biostatistics Series. New York: Taylor & Francis; 2017.
-
- Cox DR, Oakes D. Analysis of survival data. London UK: Chapman and Hall; 1984.
-
- Schoenfeld D The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika. 1981;68:316–319.
-
- Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2:93–113. - PubMed
-
- Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42:507–519. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
