Exploration of the optimal retention method in vivo for stem cell therapy: Low-intensity ultrasound preconditioning
- PMID: 40390863
- PMCID: PMC12088760
- DOI: 10.1016/j.reth.2025.04.012
Exploration of the optimal retention method in vivo for stem cell therapy: Low-intensity ultrasound preconditioning
Abstract
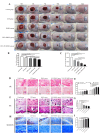
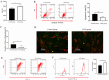
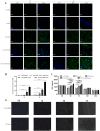
Bone marrow mesenchymal stem cells (BMSCs) are pluripotent and self-renewing, exerting a crucial role in the domain of regenerative medicine. Nevertheless, BMSCs encounter challenges such as low cell viability and inadequate homing during transplantation, thereby restricting their therapeutic efficacy. Hence, current research is concentrated on identifying optimal retention approaches following BMSCs transplantation to enhance its effectiveness. Low-intensity ultrasound (LIUS) has been verified as an efficacious method to enhance the performance of BMSCs. We established a skin trauma model and assessed the therapeutic effect of LIUS-preconditioned BMSCs. The results demonstrated that pretreatment with LIUS could expedite wound healing and effectively diminish scar formation post-transplantation by promoting proliferation capacity, reinforcing anti-apoptotic attributes, improving homing ability, and significantly enhancing the transplantation effect of BMSCs. These discoveries imply that LIUS might constitute a promising strategy for attaining optimal retention after stem cell transplantation in regenerative medicine and wound repair therapy.
Keywords: Apoptosis; Bone marrow mesenchymal stem cells; Low-intensity ultrasound; Optimal retention method; Preconditioning.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Oh E.J., Lee H.W., Kalimuthu S., Kim T.J., Kim H.M., Baek S.H., et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J Control Release. 2018;279:79–88. - PubMed
-
- Kim H.K., Lee S.G., Lee S.W., Oh B.J., Kim J.H., Kim J.A., et al. A subset of paracrine factors as efficient biomarkers for predicting vascular regenerative efficacy of mesenchymal stromal/stem cells. Stem Cells. 2018;37:77–88. - PubMed
-
- Nakamura Y., Ishikawa H., Kawai K., Tabata Y., Suzuki S. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials. 2013;34:9393–9400. - PubMed
-
- Elvasandran K., Makhoul G., Jaiswal P.K., Jurakhan R., Li L., Ridwan K., et al. Tumor necrosis factor-α and hypoxia-induced secretome therapy for myocardial repair. Ann Thorac Surg. 2017;105:715–723. - PubMed
LinkOut - more resources
Full Text Sources

