Quality of vitamin K antagonist treatment during the last year of life
- PMID: 40390869
- PMCID: PMC12087289
- DOI: 10.1002/hem3.70135
Quality of vitamin K antagonist treatment during the last year of life
Erratum in
-
Correction to "Quality of vitamin K antagonist treatment during the last year of life".Hemasphere. 2025 Jul 28;9(7):e70173. doi: 10.1002/hem3.70173. eCollection 2025 Jul. Hemasphere. 2025. PMID: 40727945 Free PMC article.
Abstract
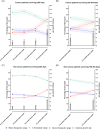
Limited data exist on the quality of anticoagulation in patients approaching the end of life. This study evaluated vitamin K antagonist (VKA) anticoagulation control during the last year of life, using nationwide data from Statistics Netherlands, linked to anticoagulation clinics' data and the Netherlands Cancer Registry. We included prevalent VKA users who were hospitalized with a severe medical condition and died between January 1, 2013, and December 31, 2019. Anticoagulation control was assessed using time in therapeutic range (TTR), time above therapeutic range (TAR), and time below therapeutic range (TBR) and the international normalized ratio (INR) variance growth rate (VGR), which reflects INR variability. Anticoagulation control was examined by two approaches: (1) over four intervals (0-12 months, 0-9 months, 0-6 months, and 0-3 months preceding death), and (2) in 3-month intervals (9-12, 6-9, 3-6, and 0-3 months before death) to describe temporal changes. Among 6874 VKA users in their last year of life (median age 82 [Interquartile range: 76-87] years, 46.9% female), the most prevalent severe medical conditions were heart disease (60.4%), cancer (16.2%), and hip fracture (15.2%). As death approached, TTR and TBR decreased, while TAR and mean VGR increased, particularly in the last 3 months of life. This decline was more pronounced in cancer patients and acenocoumarol users. In conclusion, the quality of VKA anticoagulation declined in the last year of life in severely ill patients, marked by reduced TTR and increased TAR and VGR, suggesting an increased bleeding risk. These findings highlight the importance of reassessing VKA use and considering discontinuation in patients approaching the end of life.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
C. V. has received travel support from the International Society on Thrombosis and Haemostasis (ISTH) for attending the ISTH Congress in 2024. I. M. has received research support from BMS and Pfizer, and has received speaker fees from LEO Pharma, BMS, Pfizer, and AstraZeneca, paid to her institution. S. S. has received speaker fees from Bayer, BMS, and Pfizer. S. I. R. N. has received a payment for a lecture at LEO Pharma and is a Medical Director of Thrombosis UK (non‐remunerated charity work). F. A. K. has received research support from Bayer, BMS, BSCI, AstraZeneca, MSD, LEO Pharma, Actelion, Farm‐X, The Netherlands Organization for Health Research and Development, the Dutch Thrombosis Foundation, the Dutch Heart Foundation, and the Horizon Europe Program. All support was paid to the Leiden University Medical Center. Q. C. was supported by the Chinese Government Scholarship (no. 201906380148) for his PhD study at the Leiden University Medical Center between September 2019 and September 2023, and has received travel support from the ISTH for attending the ISTH Congress between 2022 and 2024. M. J. H. A. Kruip has received speaker fees from Roche, paid to her institution. All authors declare that no known competing financial interests or personal relationships could have appeared to influence the work reported in this article.
Figures



References
-
- Joosten LPT, van Doorn S, van de Ven PM, et al. Safety of switching from a vitamin K antagonist to a non‐vitamin K antagonist oral anticoagulant in frail older patients with atrial fibrillation: results of the FRAIL‐AF Randomized Controlled Trial. Circulation. 2024;149(4):279‐289. 10.1161/circulationaha.123.066485 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
