Ultrasound-driven wireless piezoelectric hydrogel synergizes with cotransplantation of NSCs-hUCMSCs for structural and functional recovery in spinal cord injury
- PMID: 40391024
- PMCID: PMC12088769
- DOI: 10.1016/j.mtbio.2025.101805
Ultrasound-driven wireless piezoelectric hydrogel synergizes with cotransplantation of NSCs-hUCMSCs for structural and functional recovery in spinal cord injury
Abstract
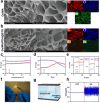
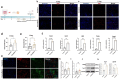
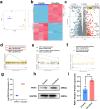
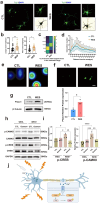
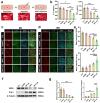
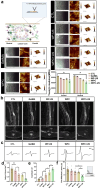
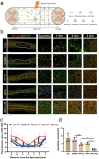
Spinal cord injury (SCI) is a devastating condition of the central nervous system, characterized by disrupted regulation of the immune microenvironment and the loss of electrical signaling, which poses significant challenges to repair. Neural stem cells (NSCs) have the potential to promote functional recovery after SCI; however, their therapeutic potential is limited by poor survival, restricted proliferation, and suboptimal differentiation. Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) possess powerful paracrine and immunomodulatory properties, providing a supportive niche that improves the engraftment and function of NSCs. Recently, piezoelectric materials have attracted increasing attention for their ability to convert mechanical energy into electrical signals, thus providing a noninvasive, wireless alternative to traditional electrode-based therapies for neural regeneration. In this study, we investigated the synergistic effects of NSCs and hUCMSCs, focusing on how hUCMSCs direct NSC differentiation and the mechanisms underlying this action. We also introduce an ultrasound-driven wireless piezoelectric hydrogel, which generates electrical signals through the piezoelectric effect. In vitro, wireless electrical stimulation activated primary cortical neurons, stimulated axonal growth, and promoted neuronal plasticity through the Piezo1 channel and downstream CREB/CAMKII signaling pathways. In a rat SCI model, wireless piezoelectric hydrogel synergized with cotransplanting NSCs-hUCMSCs and modulated the immune microenvironment during the acute phase, thereby restructuring scar cavities during the chronic phase, suppressing scar formation, accelerating neurogenesis, and facilitating axonal regeneration. These results emphasize the potential of synergizing stem cell therapies with wireless piezoelectric stimulation as a promising strategy for SCI repair, providing novel insights into the clinical translation of regenerative treatments.
Keywords: Piezoelectric nanogenerator; Spinal cord injury; Ultrasound; Wireless electrical stimulation.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Synergistic effects of human umbilical cord mesenchymal stem cells/neural stem cells and epidural electrical stimulation on spinal cord injury rehabilitation.Sci Rep. 2024 Oct 30;14(1):26090. doi: 10.1038/s41598-024-75754-x. Sci Rep. 2024. PMID: 39478010 Free PMC article.
-
Biomimetic piezoelectric hydrogel system for energy metabolism reprogramming in spinal cord injury repair.Theranostics. 2025 Mar 31;15(11):4955-4969. doi: 10.7150/thno.108329. eCollection 2025. Theranostics. 2025. PMID: 40303325 Free PMC article.
-
Release of O-GlcNAc transferase inhibitor promotes neuronal differentiation of neural stem cells in 3D bioprinted supramolecular hydrogel scaffold for spinal cord injury repair.Acta Biomater. 2022 Oct 1;151:148-162. doi: 10.1016/j.actbio.2022.08.031. Epub 2022 Aug 21. Acta Biomater. 2022. PMID: 36002129
-
Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair.Cells. 2022 Mar 1;11(5):846. doi: 10.3390/cells11050846. Cells. 2022. PMID: 35269466 Free PMC article. Review.
-
Direct neuronal differentiation of neural stem cells for spinal cord injury repair.Stem Cells. 2021 Aug;39(8):1025-1032. doi: 10.1002/stem.3366. Epub 2021 Mar 5. Stem Cells. 2021. PMID: 33657255 Review.
References
-
- Chen S.-Y., Yang R.-L., Wu X.-C., Zhao D.-Z., Fu S.-P., Lin F.-Q., Li L.-Y., Yu L.-M., Zhang Q., Zhang T. Mesenchymal stem cell transplantation: neuroprotection and nerve regeneration after spinal cord injury. J. Inflamm. Res. 2023;16:4763–4776. doi: 10.2147/JIR.S428425. PubMed. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

