The electronic nose in lung cancer diagnostics: a systematic review and meta-analysis
- PMID: 40391063
- PMCID: PMC12086828
- DOI: 10.1183/23120541.00723-2024
The electronic nose in lung cancer diagnostics: a systematic review and meta-analysis
Abstract
Background: Lung cancer remains a leading cause of cancer-related deaths. Early-stage detection is pivotal for curative interventions, yet most cases are diagnosed at advanced stages. Noninvasive point-of-care diagnostic strategies are needed to reduce lung cancer mortality rates. We assess the potential of electronic nose (eNose) devices as a tool for lung cancer detection.
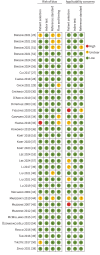
Study design and methods: All online available databases were searched. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we reviewed studies using eNose for in vivo exhaled air analysis in lung cancer patients. Included patients had pathologically confirmed lung cancer, both non-small cell lung cancer and small cell lung cancer, in various stages. Primary outcomes were sensitivity, specificity, negative predictive value and area under the curve. Secondary outcomes evaluated eNose performance in distinguishing non-small cell lung cancer subtypes and small cell lung cancer. Additionally, a subgroup analysis was conducted to evaluate the efficacy of various commonly used sensors in this differentiation. The Quality Assessment of Diagnostic Accuracy Studies 2 tool was used for risk-of-bias assessment.
Results: We included 35 studies, comprising 4483 patients. Pooled sensitivity and specificity were 0.90 (95% CI 0.87-0.93) and 0.89 (95% CI 0.85-0.93), respectively. The median negative predictive value was 0.93 and the median area under the curve was 0.91. Both the likelihood of bias and concerns regarding applicability were minimal.
Interpretation: eNose devices hold promise as noninvasive, point-of-care tools for lung cancer diagnosis. Owing to the high sensitivity and negative predictive values and great ease of use, they could be valuable additions to the diagnostic toolbox. Further research and validation efforts are critical to maximise their impact on early lung cancer detection.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: S. Kort reports support for the present study from the eNose Company and grants from eNose Company. Conflict of interest: W.H. Van Geffen reports a leadership role with the European Respiratory Society and NVALT (Dutch Society of Respiratory Physicians); and there have been trials run by his department funded by Roche and MSD. Conflict of interest: The remaining authors have nothing to disclose.
Figures


Similar articles
-
Prediction of response to anti-PD-1 therapy in patients with non-small-cell lung cancer by electronic nose analysis of exhaled breath.Ann Oncol. 2019 Oct 1;30(10):1660-1666. doi: 10.1093/annonc/mdz279. Ann Oncol. 2019. PMID: 31529107
-
Multi-centre prospective study on diagnosing subtypes of lung cancer by exhaled-breath analysis.Lung Cancer. 2018 Nov;125:223-229. doi: 10.1016/j.lungcan.2018.09.022. Epub 2018 Sep 29. Lung Cancer. 2018. PMID: 30429025
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Diagnostic performance of volatile organic compounds analysis and electronic noses for detecting colorectal cancer: a systematic review and meta-analysis.Front Oncol. 2024 May 13;14:1397259. doi: 10.3389/fonc.2024.1397259. eCollection 2024. Front Oncol. 2024. PMID: 38817891 Free PMC article.
-
Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Jul. Report No.: 14-05210-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Jul. Report No.: 14-05210-EF-1. PMID: 27583318 Free Books & Documents. Review.
References
LinkOut - more resources
Full Text Sources
