Characterisation of a GNAS variant linked to cortisol-producing adrenocortical adenoma
- PMID: 40391091
- PMCID: PMC12087278
- DOI: 10.1530/EO-25-0009
Characterisation of a GNAS variant linked to cortisol-producing adrenocortical adenoma
Abstract
Objective: Adrenocortical adenomas are frequent in the general population and can be associated with autonomous cortisol excess, increasing morbidity and mortality. Altered cAMP/PKA signalling is common in sporadic cortisol-producing adenomas, typically due to somatic activating mutations in the catalytic subunit α of PKA (PRKACA) or the G-protein α subunit, Gαs (GNAS), which activate cAMP signalling. We previously identified a novel p.Lys58Gln GNAS somatic variant in a patient with a 5.3 cm adenoma and overt Cushing's syndrome. This novel mutation was not charactersised before but provided enough evidence to warrant further investigation.
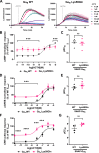
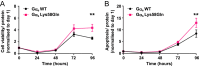
Design and methods: Using HEK293 cells depleted of GNAS, we established wild-type (WT) Gαs and Gαs-Lys58Gln stable cell lines and evaluated adrenocorticotropic hormone (ACTH) receptor signalling using a cAMP GloSensor assay, measured CREB transcription factor phosphorylation (pCREB) by AlphaLISA and assessed CRE luciferase reporter activity. Cell viability and apoptosis were also assessed over 5 days.
Results: The Gαs-Lys58Gln variant showed a significantly higher basal cAMP, pCREB and CRE luciferase reporter concentration and a greater response to ACTH (0-10 nM, P < 0.001) compared to WT Gαs. The variant had no effect on ligand potency. There was also significantly enhanced cell viability and apoptosis in cells with the Gαs-Lys58Gln variant.
Conclusions: In conclusion, our study demonstrated that the Gαs-Lys58Gln variant is associated with constitutive activation of GNAS signalling, similar to Arg201 mutations previously reported in adrenocortical adenomas, potentially representing a new pathogenic mechanism in a subset of patients with adrenal Cushing syndrome. This variant may also affect cell proliferation and requires further study.
Keywords: ACTH receptor; G protein; cAMP/PKA signalling; cortisol-producing adenomas; somatic mutation.
© the author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the work reported.
Figures

Similar articles
-
PRKACA mutations in cortisol-producing adenomas and adrenal hyperplasia: a single-center study of 60 cases.Eur J Endocrinol. 2015 Jun;172(6):677-85. doi: 10.1530/EJE-14-1113. Epub 2015 Mar 6. Eur J Endocrinol. 2015. PMID: 25750087
-
Clinical characteristics of PRKACA mutations in Chinese patients with adrenal lesions: a single-centre study.Clin Endocrinol (Oxf). 2016 Dec;85(6):954-961. doi: 10.1111/cen.13134. Epub 2016 Jul 27. Clin Endocrinol (Oxf). 2016. PMID: 27296931
-
Somatic mutations of the catalytic subunit of cyclic AMP-dependent protein kinase (PRKACA) gene in Japanese patients with several adrenal adenomas secreting cortisol [Rapid Communication].Endocr J. 2014;61(8):825-32. doi: 10.1507/endocrj.ej14-0282. Epub 2014 Jul 25. Endocr J. 2014. PMID: 25069672
-
cAMP signaling in cortisol-producing adrenal adenoma.Eur J Endocrinol. 2015 Oct;173(4):M99-106. doi: 10.1530/EJE-15-0353. Epub 2015 Jul 2. Eur J Endocrinol. 2015. PMID: 26139209 Review.
-
PRKACA: the catalytic subunit of protein kinase A and adrenocortical tumors.Front Cell Dev Biol. 2015 May 20;3:26. doi: 10.3389/fcell.2015.00026. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26042218 Free PMC article. Review.
References
-
- Almeida MQ, Azevedo MF, Xekouki P, et al. . 2012. Activation of cyclic AMP signaling leads to different pathway alterations in lesions of the adrenal cortex caused by germline PRKAR1A defects versus those due to somatic GNAS mutations. J Clin Endocrinol Metab 97 E687–E693. (10.1210/jc.2011-3000) - DOI - PMC - PubMed
-
- Bertherat J, Groussin L, Sandrini F, et al. . 2003. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res 63 5308–5319. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
