Pharmacovigilance Study on Adverse Events of Nicotine Replacement Therapy, Bupropion, and Varenicline in Patients with Chronic Obstructive Pulmonary Disease
- PMID: 40391127
- PMCID: PMC12087981
- DOI: 10.2147/COPD.S514133
Pharmacovigilance Study on Adverse Events of Nicotine Replacement Therapy, Bupropion, and Varenicline in Patients with Chronic Obstructive Pulmonary Disease
Abstract
Purpose: Chronic obstructive pulmonary disease (COPD) is one of the most prevalent respiratory disorders, with smoking being a major risk factor. Smoking cessation is therefore crucial in the management of COPD. This study aimed to comprehensively evaluate the safety profiles of common cessation therapies, including nicotine replacement therapy, bupropion, and varenicline.
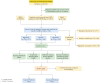
Patients and methods: Using the FDA Adverse Event Reporting System (FAERS) database from Q1 2004 to Q2 2024, we analyzed adverse events (AEs) associated with bupropion, nicotine, and varenicline in COPD patients. Disproportionality analysis, case-by-case evaluation, and co-medication analysis were performed to identify positive safety signals.
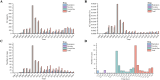
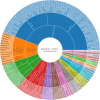
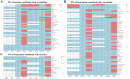
Results: Eighty-eight positive safety signals were identified, primarily involving psychiatric, nervous system, and gastrointestinal disorders. Notable AEs included depression, nausea, anxiety, abnormal dreams, and insomnia. Critically, eight PTs indicated serious AEs associated with psychiatric disorders that were not present in the labeling but required Important Medical Event (IME) surveillance. Experiencing severe neuropsychiatric symptoms (eg, suicidal thoughts and suicide attempts) was the major reason for limiting the use of these drugs, especially varenicline, for which the FDA issued a black box warning in 2009. Nicotine combined with varenicline showed higher risks for skin reactions and gastrointestinal issues. Most AEs occurred within the first 30 days of therapy, with some persisting beyond a year.
Conclusion: This study highlights significant psychiatric, neurological, and gastrointestinal AEs associated with smoking cessation therapies in patients with COPD. Clinicians are advised to be particularly cautious of these risks, especially when using combination therapies or treating patients with a predisposition to psychiatric disorders.
Keywords: FAERS database; bupropion; chronic obstructive pulmonary disease; nicotine; varenicline.
© 2025 Wang et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

