Huashi Jiedu Decoction Enhances 5-Fluorouracil Efficacy in Gastric Cancer via miRNA-21-3p/p53 Pathway
- PMID: 40391175
- PMCID: PMC12087606
- DOI: 10.2147/DDDT.S513371
Huashi Jiedu Decoction Enhances 5-Fluorouracil Efficacy in Gastric Cancer via miRNA-21-3p/p53 Pathway
Abstract
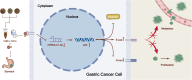
Purpose: To explore the mechanism of Huashi Jiedu Decoction (HJD) synergizing with 5-fluorouracil (5-Fu) in gastric cancer (GC) therapy.
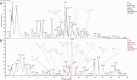
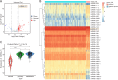
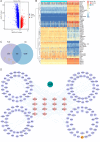
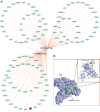
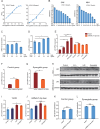
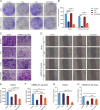
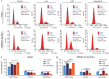
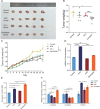
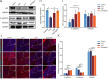
Methods: MicroRNAs (miRNAs) and genes involved in HJD-mediated GC treatment were identified using ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry, network pharmacology, Gene Ontology, Kyoto Encyclopedia of Genes and Genomes, and molecular docking. The effects of HJD on 5-Fu sensitivity in BGC-823 cells were evaluated with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays, reverse transcription quantitative polymerase chain reaction (RT-qPCR), and Western blotting. Synergistic effects in vector-transfected and miRNA-21-3p knockdown cells were assessed by colony formation, wound healing, transwell assays, and flow cytometry (FCM). An in vivo study evaluated the impact of HJD on 5-Fu sensitivity, measuring miRNA-21-3p, tumor protein p53 (p53), N-cadherin, vimentin, and E-cadherin in tumors, along with tumor volume and weight.
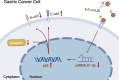
Results: miRNA-21-3p and p53 were key targets in HJD's therapeutic effect on GC. RT-qPCR showed that HJD combined with 5-Fu reduced miRNA-21-3p and upregulated p53 in vector cells and increased p53 mRNA (p < 0.01) and protein (p < 0.05) compared to 5-Fu alone. These effects were abolished in miRNA-21-3p knockdown cells. The combination reduced colony formation by 48.92% (p < 0.01), suppressed transwell migration by 28.5% (p < 0.01), and inhibited wound healing by 81.9% compared to 5-Fu monotherapy (p < 0.001), with no effects in knockdown cells. FCM showed a 15.1% increase in G0/G1 phase arrest (p < 0.05). In vivo, the combination significantly reduced tumor volume (p < 0.05) and weight by 18.7%, with concomitant miRNA-21-3p downregulation (p < 0.0001), EMT marker suppression (N-cadherin, vimentin), and tumor suppressor activation (p53, E-cadherin) versus 5-Fu alone.
Conclusion: HJD enhances 5-Fu's effects on GC by regulating the miRNA-21-3p/p53 pathway and modulating cadherin expression, supporting its potential as an adjunctive treatment in GC.
Keywords: 5-fluorouracil; gastric cancer; huashi jiedu decoction; miRNAs; p53.
© 2025 Hong et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
The Mechanism of Sijunzi Decoction Suppresses Gastric Cancer Metastasis via the m6A Methyltransferase METTL14 Based on Untargeted Metabolomics Studies and Network Pharmacology Analysis.Drug Des Devel Ther. 2025 Mar 31;19:2369-2392. doi: 10.2147/DDDT.S506702. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40190808 Free PMC article.
-
Rosmarinic acid reduces the resistance of gastric carcinoma cells to 5-fluorouracil by downregulating FOXO4-targeting miR-6785-5p.Biomed Pharmacother. 2019 Jan;109:2327-2334. doi: 10.1016/j.biopha.2018.10.061. Epub 2018 Nov 30. Biomed Pharmacother. 2019. PMID: 30551491
-
Gypenosides Synergistically Enhances the Anti-Tumor Effect of 5-Fluorouracil on Colorectal Cancer In Vitro and In Vivo: A Role for Oxidative Stress-Mediated DNA Damage and p53 Activation.PLoS One. 2015 Sep 14;10(9):e0137888. doi: 10.1371/journal.pone.0137888. eCollection 2015. PLoS One. 2015. PMID: 26368019 Free PMC article.
-
18β-glycyrrhetinic acid promotes gastric cancer cell autophagy and inhibits proliferation by regulating miR-328-3p/signal transducer and activator of transcription 3.World J Gastroenterol. 2023 Jul 21;29(27):4317-4333. doi: 10.3748/wjg.v29.i27.4317. World J Gastroenterol. 2023. PMID: 37545635 Free PMC article.
-
Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway.Invest New Drugs. 2020 Apr;38(2):329-339. doi: 10.1007/s10637-019-00781-9. Epub 2019 May 17. Invest New Drugs. 2020. PMID: 31102118
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

