The MVA-VP2-NS1-2A-NS2-Nt vaccine candidate provides heterologous protection in sheep against bluetongue virus
- PMID: 40391210
- PMCID: PMC12086148
- DOI: 10.3389/fimmu.2025.1566225
The MVA-VP2-NS1-2A-NS2-Nt vaccine candidate provides heterologous protection in sheep against bluetongue virus
Abstract
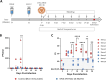
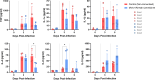
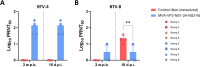
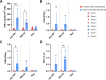
Bluetongue (BT) is an important arthropod-borne livestock disease transmitted by Culicoides midges. The etiological agent, Bluetongue virus (BTV), can lead to severe economic losses due to reduced productivity and trade restrictions. Nowadays, classical vaccines based on inactivated viruses are used to control outbreaks but do not confer multiserotype protection, which reinforces the idea of pursuing research into developing strategies that enhance the immune response directed to conserved antigenic regions, aiming broader protection across multiple serotypes. Recently, we described a vaccine candidate that confers full protection against a homologous serotype of BTV based on recombinant Modified Vaccinia Virus Ankara (MVA) co-expressing the highly conserved BTV nonstructural protein NS1 and the N-terminal end of NS2 along with protein VP2 of BTV-4. In this work, we evaluated the multiserotype protective capacity of this recombinant vaccine candidate in sheep after infection with the heterologous virus BTV-8, achieving a significant blockade of viral replication and attenuation of the clinical signs induced by BTV. After infection, vaccinated animals showed more regulated pro-inflammatory cytokine levels compared to non-vaccinated sheep. In addition, we noticed the induction of potent T cell immune responses specific to NS1 and NS2-Nt proteins of BTV, mainly based on CD8+ T cells, which could mediate the protection against BTV-8. Moreover, stimulated immunized sheep PBMCs with BTV antigens triggered the secretion of IL-6, IL-1β, IL-1α, IL-17a, IL-10 and IFN-γ, cytokines that play crucial roles in initiating immune responses.
Keywords: DIVA; MVA; bluetongue virus (BTV); multiserotype; orbivirus; sheep; vaccine.
Copyright © 2025 Jiménez-Cabello, Utrilla-Trigo, Illescas-Amo, Rodríguez-Sabando, Benavides-Silván, Calvo-Pinilla and Ortego.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Co-expression of VP2, NS1 and NS2-Nt proteins by an MVA viral vector induces complete protection against bluetongue virus.Front Immunol. 2024 Jul 12;15:1440407. doi: 10.3389/fimmu.2024.1440407. eCollection 2024. Front Immunol. 2024. PMID: 39072326 Free PMC article.
-
The Combined Expression of the Nonstructural Protein NS1 and the N-Terminal Half of NS2 (NS21-180) by ChAdOx1 and MVA Confers Protection against Clinical Disease in Sheep upon Bluetongue Virus Challenge.J Virol. 2022 Feb 9;96(3):e0161421. doi: 10.1128/JVI.01614-21. Epub 2021 Nov 17. J Virol. 2022. PMID: 34787454 Free PMC article.
-
CD8 T Cell Responses to an Immunodominant Epitope within the Nonstructural Protein NS1 Provide Wide Immunoprotection against Bluetongue Virus in IFNAR-/- Mice.J Virol. 2018 Jul 31;92(16):e00938-18. doi: 10.1128/JVI.00938-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29875250 Free PMC article.
-
Recombinant vaccines against bluetongue virus.Virus Res. 2014 Mar;182:78-86. doi: 10.1016/j.virusres.2013.11.013. Epub 2013 Nov 25. Virus Res. 2014. PMID: 24287057 Review.
-
Vaccines for Prevention of Bluetongue and Epizootic Hemorrhagic Disease in Livestock: A North American Perspective.Vector Borne Zoonotic Dis. 2015 Jun;15(6):385-96. doi: 10.1089/vbz.2014.1698. Vector Borne Zoonotic Dis. 2015. PMID: 26086559 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

