Itolizumab regulates activating and inhibitory signals on effector cells, improving their cytotoxicity against CD318+ tumor cell lines
- PMID: 40391211
- PMCID: PMC12086168
- DOI: 10.3389/fimmu.2025.1585597
Itolizumab regulates activating and inhibitory signals on effector cells, improving their cytotoxicity against CD318+ tumor cell lines
Abstract
Introduction: The CD6-CD318 axis has emerged as a potential target for immuno-oncology. Recent work has shown that blocking the CD6-CD318 interaction with a murine anti-human CD6 antibody increases lymphocyte cytotoxicity. However, several studies have demonstrated the drawbacks associated with the clinical use of murine antibodies and the variability among anti-CD6 antibodies. Therefore, evidence that the first-in-class humanized anti-human CD6 antibody itolizumab could be used for cancer immunotherapy may be a breakthrough in developing an antitumor clinical approach.
Methods: Phenotypic and functional characterization of peripheral blood mononuclear cells (PBMCs) from healthy donors after challenge with CD318+ cell lines was performed by flow cytometry. In addition, IFNγ was determined by ELISA in culture supernatants. Immunohistochemical analyses of breast tumor samples were also performed.
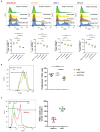
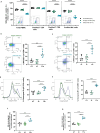
Results and discussion: Here, we provide evidence supporting the rationale for itolizumab in cancer immunotherapy. The blockade of the CD6-CD318 interaction by itolizumab increases the cytotoxic capacity of CD8 T and NK cells over CD318+ tumor lines, reverses the NKG2A/NKG2D ratio, and increases granzyme B and IFNγ production. Itolizumab also regulates immune responses by downregulating CD5 expression and upregulating PD-1 and CTLA-4 inhibitory receptors on lymphocytes, which contribute to reducing exacerbated responses and additively enhancing CD318+ tumor cell cytotoxicity when combined with other immunocheckpoint inhibitors. In addition, we report that CD6-CD318 interaction inhibits lymphocyte proliferation and survival while downregulating CD6 expression on lymphocytes in vitro and in human breast cancer tissue samples, reinforcing the role of the CD6-CD318 axis as an immune checkpoint and highlighting the potential of itolizumab as an immune checkpoint inhibitor. Taken together, our results provide the first evidence linking the blocking of the CD6-CD318 axis by itolizumab with the potentiation of functional properties of lymphocytes, highlighting itolizumab as a novel promising immunotherapy for CD318+ tumors and supporting the relevance of new combinatorial therapies with checkpoint inhibitors.
Keywords: ALCAM(CD166); CD318; CD5; CD6; CD8 lymphocytes; NK cells; immune checkpoint.
Copyright © 2025 González Muñoz, Álvarez Arzola, Calvo Pérez, Frometa Campanón, Hernández Casaña, Fernández-Calienes Valdés, Lorenzo-Luaces, Mazorra Herrera, Crombet Ramos and Labrada Mon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
CD6 is a target for cancer immunotherapy.JCI Insight. 2021 Mar 8;6(5):e145662. doi: 10.1172/jci.insight.145662. JCI Insight. 2021. PMID: 33497367 Free PMC article.
-
Activation of cytotoxic lymphocytes through CD6 enhances killing of cancer cells.Cancer Immunol Immunother. 2024 Jan 27;73(2):34. doi: 10.1007/s00262-023-03578-1. Cancer Immunol Immunother. 2024. PMID: 38280067 Free PMC article.
-
Activation of Cytotoxic Lymphocytes Through CD6 Enhances Killing of Cancer Cells.Res Sq [Preprint]. 2023 Oct 9:rs.3.rs-3405677. doi: 10.21203/rs.3.rs-3405677/v1. Res Sq. 2023. Update in: Cancer Immunol Immunother. 2024 Jan 27;73(2):34. doi: 10.1007/s00262-023-03578-1. PMID: 37886483 Free PMC article. Updated. Preprint.
-
Therapeutic Targeting of CD6 in Autoimmune Diseases: A Review of Cuban Clinical Studies with the Antibodies IOR-T1 and Itolizumab.Curr Drug Targets. 2016;17(6):666-77. doi: 10.2174/1389450117666160201114308. Curr Drug Targets. 2016. PMID: 26844560 Review.
-
Clinical and experimental evidence for targeting CD6 in immune-based disorders.Autoimmun Rev. 2018 May;17(5):493-503. doi: 10.1016/j.autrev.2017.12.004. Epub 2018 Mar 9. Autoimmun Rev. 2018. PMID: 29526637 Review.
Cited by
-
Differential Role of CD318 in Tumor Immunity Affecting Prognosis in Colorectal Cancer Compared to Other Adenocarcinomas.J Clin Med. 2025 Jul 19;14(14):5139. doi: 10.3390/jcm14145139. J Clin Med. 2025. PMID: 40725832 Free PMC article.
References
-
- Braun M, Muller B, ter Meer D, Raffegerst S, Simm B, Wilde S, et al. . The CD6 scavenger receptor is differentially expressed on a CD56 natural killer cell subpopulation and contributes to natural killer-derived cytokine and chemokine secretion. J Innate Immun. (2011) 3:420–34. doi: 10.1159/000322720 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

