Protective effects of Nippostrongylus brasiliensis- derived uridine via the apical sodium-dependent bile acid transporter in a mouse model of TNBS-induced inflammatory bowel disease
- PMID: 40391223
- PMCID: PMC12087013
- DOI: 10.3389/fimmu.2025.1600838
Protective effects of Nippostrongylus brasiliensis- derived uridine via the apical sodium-dependent bile acid transporter in a mouse model of TNBS-induced inflammatory bowel disease
Abstract
Introduction: Inflammatory bowel disease (IBD), a chronic immune-mediated gastrointestinal disorder mainly covering Crohn's Disease and Ulcerative Colitis, has an unclear etiology. The exploration of novel intervention strategies remains a key scientific issue that is urgently needed for IBD treatment. The hygiene hypothesis has led researchers to notice that worm infections can regulate the immune system, which might help treat inflammatory diseases. Nippostrongylus brasiliensis (Nb), similar to human hookworms in life cycle and symptoms, is often used in hookworm research. Our previous study also demonstrated that Nb-derived uridine screened from ES could exert anti-inflammatory and anti-atherosclerotic effects.
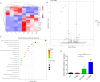
Methods: In this study, we established the protective and anti-inflammation effect of Nb infection and ES intervention in TNBS-induced IBD model in mice and further validated the efficiency of uridine screened from ES. Moreover, we conducted an RNA sequencing (RNA-Seq) analysis to elucidate the relevant possible functional mechanisms responsible for the protective and anti-inflammation effects of ES or uridine administration.
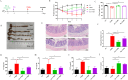
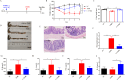
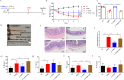
Results: Current results have demonstrated that uridine can exhibit a protective effect on TNBS-induced IBD in mice. Moreover, it was identified that slc10a2 exhibited high expression after uridine intervention. By specific inhibition of the encoding protein (ASBT), its impact on the protective efficacy has been interrupted.
Discussion: The current study has illustrated that uridine is capable of exerting potential therapeutic and anti-inflammatory effects on Inflammatory Bowel Disease (IBD) by modulating slc10a2. These findings could offer a novel therapeutic target for the intervention of IBD.
Keywords: Nippostrongylus brasiliensis; RNA sequencing; apical sodium-dependent bile acid transporter; inflammatory bowel disease; uridine.
Copyright © 2025 Yuan, Wang, Chen, Ding, Zhang, Yao, Zhang, Dai and Bai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model.Biomolecules. 2024 Jun 8;14(6):672. doi: 10.3390/biom14060672. Biomolecules. 2024. PMID: 38927075 Free PMC article.
-
[Nippostrongylus brasiliensis alleviates dextran sulfate sodium salt-induced ulcerative colitis in mice: a preliminary study].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2024 Aug 29;36(5):450-459. doi: 10.16250/j.32.1374.2024073. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2024. PMID: 39623985 Chinese.
-
Inflammatory bowel disease alters intestinal bile acid transporter expression.Drug Metab Dispos. 2014 Sep;42(9):1423-31. doi: 10.1124/dmd.114.058065. Epub 2014 Jun 25. Drug Metab Dispos. 2014. PMID: 24965812
-
Inflammatory Bowel Disease: A Review of Pre-Clinical Murine Models of Human Disease.Int J Mol Sci. 2022 Aug 19;23(16):9344. doi: 10.3390/ijms23169344. Int J Mol Sci. 2022. PMID: 36012618 Free PMC article. Review.
-
IBD and Bile Acid Absorption: Focus on Pre-clinical and Clinical Observations.Front Physiol. 2020 Jun 12;11:564. doi: 10.3389/fphys.2020.00564. eCollection 2020. Front Physiol. 2020. PMID: 32595517 Free PMC article. Review.
References
-
- Collaborators, G.I.B.D . The global, regional, and national burden of inflammatory ibowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2019) 5:17–30. doi: 10.1016/S2468-1253(19)30333-4 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

