Lung Epithelial Cell Membrane-Camouflaged ROS-Activatable Berberine Nanoparticles for Targeted Treatment in Acute Lung Injury
- PMID: 40391301
- PMCID: PMC12087599
- DOI: 10.2147/IJN.S514611
Lung Epithelial Cell Membrane-Camouflaged ROS-Activatable Berberine Nanoparticles for Targeted Treatment in Acute Lung Injury
Abstract
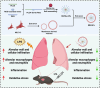
Introduction: Acute lung injury (ALI) seriously threatens human health and is induced by multiple factors. When ALI occurs, lung lesions affect gas exchange and may trigger respiratory failure. Current clinical treatments are limited, and traditional drug delivery has drawbacks. Berberine, a natural drug with anti-inflammatory effects, has difficulty in effectively exerting its efficacy.
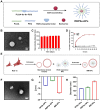
Methods: The study designed a nano-micelle. Hydrophobic berberine was encapsulated with diselenide bonds as the linker. Then, lung epithelial cell membranes were extracted to encapsulate and disguise the nano-micelle. These nanoparticles were injected intravenously. Thanks to the cell membrane's specificity, they could bind to lung tissue, achieving targeted lung delivery. In the inflamed area of acute lung injury, the significantly increased reactive oxygen species level was used to break the diselenide bonds, enabling precise berberine release at the lung injury site.
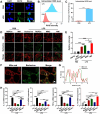
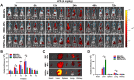
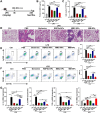
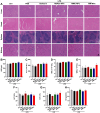
Results: The nano-drug (MM-NPs) was successfully prepared, with the encapsulation efficiency of berberine in the micelles reaching 68.2%. In a ROS environment, the nano-micelles could quickly release over 80% of berberine. In inflammatory MLE-12 cells, MM-NPs responded well to ROS, and cellular inflammatory factor levels were significantly improved after treatment. In a lipopolysaccharide (LPS)-induced pneumonia mouse model, MM-NPs achieved lung targeting. Further studies showed that MM-NPs administration significantly alleviated LPS-induced lung injury in mice. Additionally, evaluation indicated MM-NPs had good in-vivo safety with no obvious adverse reactions.
Conclusion: This study successfully developed a novel delivery system, MM-NPs, overcoming berberine's low bioavailability problem in treating acute lung injury. The system has excellent physicochemical properties, biocompatibility, and metabolic safety. In vitro and animal experiments verified it can significantly enhance the therapeutic effect, offering new ideas and hopes for acute lung injury treatment. In the future, clinical trials can be advanced, and new lung targeting strategies explored for more therapeutic breakthroughs.
Keywords: acute lung injury; berberine; lung-targeted delivery; nano-micelles; reactive oxygen species.
© 2025 Jin et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Synergistic anti-oxidant and anti-inflammatory effects of ceria/resatorvid co-decorated nanoparticles for acute lung injury therapy.J Nanobiotechnology. 2023 Dec 21;21(1):502. doi: 10.1186/s12951-023-02237-y. J Nanobiotechnology. 2023. PMID: 38129906 Free PMC article.
-
Mitochondria-Modulating Porous Se@SiO2 Nanoparticles Provide Resistance to Oxidative Injury in Airway Epithelial Cells: Implications for Acute Lung Injury.Int J Nanomedicine. 2020 Mar 31;15:2287-2302. doi: 10.2147/IJN.S240301. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32280221 Free PMC article.
-
Platelet membrane-cloaked biomimetic nanoparticles for targeted acute lung injury therapy.Colloids Surf B Biointerfaces. 2025 Jun;250:114542. doi: 10.1016/j.colsurfb.2025.114542. Epub 2025 Jan 30. Colloids Surf B Biointerfaces. 2025. PMID: 39893893
-
Ameliorating effects of berberine on sepsis-associated lung inflammation induced by lipopolysaccharide: molecular mechanisms and preclinical evidence.Pharmacol Rep. 2023 Aug;75(4):805-816. doi: 10.1007/s43440-023-00492-2. Epub 2023 May 15. Pharmacol Rep. 2023. PMID: 37184743 Review.
-
Transforming Cancer Treatment with Nanotechnology: The Role of Berberine as a Star Natural Compound.Int J Nanomedicine. 2024 Aug 22;19:8621-8640. doi: 10.2147/IJN.S469350. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39188860 Free PMC article. Review.
Cited by
-
Recent Progress in Nano-TCM Active Ingredient Co-Delivery Systems for Inflammation-Mediated Diseases.Int J Nanomedicine. 2025 Aug 2;20:9573-9596. doi: 10.2147/IJN.S526731. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40771757 Free PMC article. Review.
References
-
- Sapoznikov A, Gal Y, Falach R, et al. Early disruption of the alveolar-capillary barrier in a ricin-induced ARDS mouse model: neutrophil-dependent and -independent impairment of junction proteins. Am J Physiol Lung Cell Mol Physiol. 2019;316(1):L255–L268. doi:10.1152/ajplung.00300.2018 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

