Real-life efficacy and safety of oral propranolol for ocular adnexal infantile hemangiomas: observational cohort study
- PMID: 40391317
- PMCID: PMC12086078
- DOI: 10.3389/fopht.2025.1493171
Real-life efficacy and safety of oral propranolol for ocular adnexal infantile hemangiomas: observational cohort study
Abstract
Objective: To assess the effectiveness and safety of oral propranolol for the treatment of ocular adnexal infantile hemangiomas.
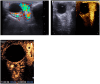
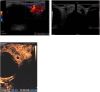
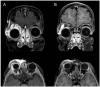
Patients and methods: retrospective observational cohort study. Propranolol was administered at an initial oral dose of 1 mg/kg and subsequently increased to 2 mg/kg for 1 year. Outcomes were evaluated by comparing pre- and post-treatment clinical findings, contrast-enhanced ultrasound (CEUS) findings and/or orbital magnetic resonance imaging findings from baseline to 3, 6, 9, 12, 24, and 48 weeks. Regression was graded as follows: satisfactory when 90% and above of the baseline lesion volume and extension decreased, acceptable when 50 to 90%, mediocre when 30 to 50%, poor less than 30%.
Results: Twenty-four patients were included in this study. The mean age at presentation was 4 ± 1 week. Sixteen (71%) patients were females and 7 (29%) were males. The mean follow-up duration was 18 ± 3 months. Therapy was started for of 23/24 patients at 5 weeks old, of 1/24 started at 9 weeks of age. The median age was 5,16 weeks. Sixteen patients (66%) had satisfactory resolution between 3 and 6 weeks, 5 (20%) after 9 weeks, and 3 (12%) after 12 weeks. One patient (5%) had a mediocre response after 24 weeks. One patient withdrew from therapy because of hypoglycemia, which was successfully managed as an outpatient. No significant adverse reactions, such as bradycardia, hypotension, bronchospasm, or congestive heart failure, were detected in this cohort.
Conclusion: This study indicates that the real-life use of oral propranolol for infantile hemangioma yields a high success rate with a lower morbidity than previously reported, particularly when managed by a proficient and multidisciplinary team.
Keywords: amblyopia; infantile hemangiomas; ocular adnexal; oral propranolol; vascular tumor.
Copyright © 2025 Malvindi, Sammarco, Elefante, Lanni, Cicala, Esposito, Picardi, Iuliano, Cohen, Mariniello, D’Aponte, Costagliola, Briganti and Strianese.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Propranolol for Treatment of Genital Infantile Hemangioma.J Urol. 2016 Mar;195(3):731-7. doi: 10.1016/j.juro.2015.09.069. Epub 2015 Sep 21. J Urol. 2016. PMID: 26400030
-
Monitoring oral propranolol for infantile hemangiomata.Dermatol Ther. 2022 Nov;35(11):e15870. doi: 10.1111/dth.15870. Epub 2022 Oct 11. Dermatol Ther. 2022. PMID: 36177767 Free PMC article.
-
[Treatment of infantile hemangiomas with low-dose propranolol: evaluation of short-term efficacy and safety].Zhonghua Yi Xue Za Zhi. 2009 Dec 1;89(44):3130-4. Zhonghua Yi Xue Za Zhi. 2009. PMID: 20193276 Chinese.
-
Infantile Hemangioma: An Updated Review.Curr Pediatr Rev. 2021;17(1):55-69. doi: 10.2174/1573396316666200508100038. Curr Pediatr Rev. 2021. PMID: 32384034 Review.
-
Propranolol treatment in life-threatening airway hemangiomas: a case series and review of literature.Int J Pediatr Otorhinolaryngol. 2013 Nov;77(11):1791-800. doi: 10.1016/j.ijporl.2013.08.011. Epub 2013 Aug 22. Int J Pediatr Otorhinolaryngol. 2013. PMID: 24074695 Review.
References
LinkOut - more resources
Full Text Sources

