This is a preprint.
Granuloma Dual RNA-Seq Reveals Composite Transcriptional Programs Driven by Neutrophils and Necrosis within Tuberculous Granulomas
- PMID: 40391323
- PMCID: PMC12087985
- DOI: 10.1101/2025.04.26.650783
Granuloma Dual RNA-Seq Reveals Composite Transcriptional Programs Driven by Neutrophils and Necrosis within Tuberculous Granulomas
Abstract
Mycobacterial granulomas lie at the center of tuberculosis (TB) pathogenesis and represent a unique niche where infecting bacteria survive in nutrient-restricted conditions and in the face of a host immune response. The granuloma's necrotic core, where bacteria reside extracellularly in humans, is difficult to assess in many experimentally tractable models. Here, using necrotic mycobacterial granulomas in adult zebrafish, we develop dual RNA-seq across different host genotypes to identify the transcriptional alterations that enable bacteria to survive within this key microenvironment. Through pharmacological and genetic interventions, we find that neutrophils within mature, necrotic granulomas promote bacterial growth, in part through upregulation of the bacterial devR regulon. We identify conserved suites of bacterial transcriptional programs induced only in the context of this unique necrotic extracellular niche, including bacterial modules related to K+ transport and rpf genes. Analysis of Mycobacterium tuberculosis strains across diverse lineages and human populations suggests that granuloma-specific transcriptional modules are targets for bacterial genetic adaptation in the context of human infection.
Figures
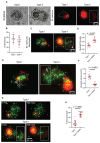


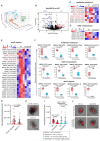
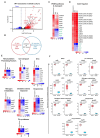
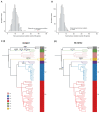
References
-
- Pagán A. J., Ramakrishnan L., The formation and function of granulomas. Annu Rev Immunol 36, 639–665 (2018). - PubMed
-
- Coleman M. T., Chen R. Y., Lee M., Lin P. L., Dodd L. E., Maiello P., Via L. E., Kim Y., Marriner G., Dartois V., Scanga C., Janssen C., Wang J., Klein E., Cho S. N., Barry C. E. 3rd, Flynn J. L., PET/CT imaging reveals a therapeutic response to oxazolidinones in macaques and humans with tuberculosis. Sci Transl Med 6, (2014). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
