VaMIEL1-mediated ubiquitination of VaMYB4a orchestrates cold tolerance through integrated transcriptional and oxidative stress pathways in grapevine
- PMID: 40391385
- PMCID: PMC12087447
- DOI: 10.1093/hr/uhaf093
VaMIEL1-mediated ubiquitination of VaMYB4a orchestrates cold tolerance through integrated transcriptional and oxidative stress pathways in grapevine
Abstract
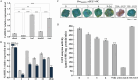
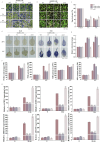
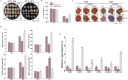
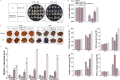
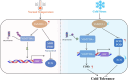
Cold stress poses a significant threat to viticulture, particularly under the increasing pressures of climate change. In this study, we identified VaMIEL1, a RING-type E3 ubiquitin ligase from Vitis amurensis, as a negative regulator of cold tolerance. Under normal temperature conditions, VaMIEL1 facilitates the ubiquitination and subsequent proteasomal degradation of the cold-responsive transcription factor VaMYB4a, thereby attenuating its regulatory role in the CBF-COR signaling cascade. However, under cold stress, VaMIEL1 expression is downregulated, leading to the stabilization of VaMYB4a and the activation of CBF-COR signaling. Through a combination of biochemical assays and functional analysis in Arabidopsis thaliana and grapevine calli, we demonstrate that VaMIEL1 overexpression reduces cold tolerance, as evidenced by increased oxidative stress, excessive reactive oxygen species (ROS) accumulation, and downregulated expression of cold-responsive genes. Conversely, silencing of VaMIEL1 enhances cold tolerance by stabilizing VaMYB4a and boosting antioxidant defenses. These findings uncover a previously unrecognized regulatory mechanism by which VaMIEL1 modulates cold tolerance through transcriptional and oxidative stress pathways, offering potential targets for the development of climate-resilient grapevine cultivars and other crops.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kerbler SM, Wigge PA. Temperature sensing in plants. Annu Rev Plant Biol. 2023;74:341–66 - PubMed
-
- Kidokoro S, Shinozaki K, Yamaguchi Shinozaki K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022;27:922–35 - PubMed
-
- Gong Z, Xiong L, Shi H. et al. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63:635–74 - PubMed
LinkOut - more resources
Full Text Sources

