Diagnosis of Plasmodium falciparum malaria at very low parasitaemias using a commercially available LAMP assay and RDT
- PMID: 40391391
- PMCID: PMC12486384
- DOI: 10.1093/trstmh/traf050
Diagnosis of Plasmodium falciparum malaria at very low parasitaemias using a commercially available LAMP assay and RDT
Abstract
Background: Malaria is the most common tropical infection in the UK. Current guidelines suggest that testing on 3 consecutive days is required following an initial negative result. This study aimed to see whether newer diagnostics (loop-mediated amplification assay [LAMP]) had sufficient sensitivity to support a change in diagnostic practice.
Methods: Blood samples from 11 participants who had undergone controlled human malaria infection (CHMI) with Plasmodium falciparum malaria were assessed from day 6 (C+6) for malaria positivity using the Carestart Malaria rapid diagnostic test (RDT) and from C+4 using the Alethia Malaria LAMP assay. Quantitative polymerase chain reaction had been performed twice daily during CHMI follow-up. A retrospective analysis of samples submitted to the Sheffield Teaching Hospitals for malaria testing over a 5-y period was conducted, evaluating the combination of the Carestart RDT alongside blood film analysis, as per UK guidelines.
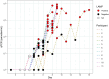
Results: In CHMI samples, LAMP was positive for all parasitaemias >1000 parasites/ml, whereas RDTs were less reliable (59% positive for parasitaemias >1000 parasites/ml). The combination of RDT and blood films for clinical samples diagnosed most infections, but only a minority of negative samples had subsequent tests.
Conclusions: LAMP has higher sensitivity than current UK recommended methods, with a potential to review the requirement for additional days of testing in the majority of patients.
Keywords: LAMP; RDT; diagnostics; malaria.
© The Author(s) 2025. Published by Oxford University Press on behalf of Royal Society of Tropical Medicine and Hygiene.
Conflict of interest statement
None declared.
Figures

References
-
- Public Health England . Malaria imported into the United Kingdom: 2019. London: Public Health England; 2019.
-
- World Health Organization . World malaria report 2022. Geneva: World Health Organization; 2022.
-
- World Health Organization . World malaria report 2017. Geneva: World Health Organization; 2017.
-
- Rogers CL, Bain BJ, Garg M et al. British Society for Haematology guidelines for the laboratory diagnosis of malaria. Br J Haematol. 2022;197(3):271–82. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

