SMYD2 Promotes Renal Tubular Cell Apoptosis and Chronic Kidney Disease Following Cisplatin Nephrotoxicity
- PMID: 40391402
- PMCID: PMC12090038
- DOI: 10.1096/fj.202402703R
SMYD2 Promotes Renal Tubular Cell Apoptosis and Chronic Kidney Disease Following Cisplatin Nephrotoxicity
Abstract
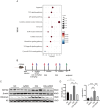
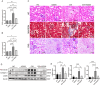
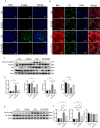
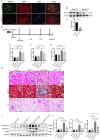
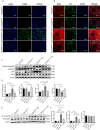
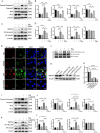
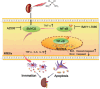
The protein lysine methyltransferase 2 (SMYD2) can affect cell proliferation, differentiation, and survival through methylation of its histone and non-histone substrates. SMYD2 has been shown to act as an oncogene to promote disease progression in a variety of cancer diseases, but its role in chronic kidney diseases (CKD) pathogenesis has not been fully elucidated. This study aims to investigate the effect of SMYD2 on cisplatin-induced CKD and its underlying mechanisms. In this study, we found that cisplatin caused severe renal injury in mice, which was accompanied by the up-regulation of SMYD2 expression. AZ505 treatment significantly down-regulated cisplatin-induced renal injury and fibrosis. It also alleviated renal apoptosis and inhibited the phosphorylation level of NF-κB p65. Conditional knockdown of Smyd2 achieved similar effects as AZ505. In renal tubular epithelial cells, inhibition or silencing of SMYD2 down-regulated cisplatin-induced apoptotic response, while overexpression of SMYD2 induced apoptotic response and activated NF-κB in response to the up-regulation of SMYD2 expression. Up-regulation of SMYD2 induced interaction and phosphorylation of SMYD2 and NF-κB p65, and inhibition of NF-κB activation further suppressed cisplatin-induced NF-κB activation and apoptosis. The present study suggests that up-regulation of SMYD2 expression in cisplatin-induced CKD may promote apoptosis of renal tubular epithelial cells and accelerate the process of renal injury through NF-κB activation. SMYD2 may serve as a potential target for effective CKD treatment.
Keywords: NF‐κB signal path way; apoptosis; renal tubular epithelial cells; the protein lysine methyltransferase 2.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Critical roles of SMYD2 lysine methyltransferase in mediating renal fibroblast activation and kidney fibrosis.FASEB J. 2021 Jul;35(7):e21715. doi: 10.1096/fj.202000554RRR. FASEB J. 2021. PMID: 34143514 Free PMC article.
-
Pharmacological inhibition of SMYD2 protects against cisplatin-induced acute kidney injury in mice.Front Pharmacol. 2022 Aug 15;13:829630. doi: 10.3389/fphar.2022.829630. eCollection 2022. Front Pharmacol. 2022. PMID: 36046818 Free PMC article.
-
Lysine methyltransferase SMYD2 promotes triple negative breast cancer progression.Cell Death Dis. 2018 Feb 27;9(3):326. doi: 10.1038/s41419-018-0347-x. Cell Death Dis. 2018. PMID: 29487338 Free PMC article.
-
Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity.Autophagy. 2008 Jul;4(5):710-2. doi: 10.4161/auto.6309. Epub 2008 May 20. Autophagy. 2008. PMID: 18497570 Review.
-
A comparative review of murine models of repeated low-dose cisplatin-induced chronic kidney disease.Lab Anim (NY). 2025 Feb;54(2):42-49. doi: 10.1038/s41684-024-01504-1. Epub 2025 Jan 30. Lab Anim (NY). 2025. PMID: 39885282 Review.
Cited by
-
Lysine Methyltransferases SMYD2 and SMYD3: Emerging Targets in Kidney Diseases.Kidney Dis (Basel). 2025 Jul 1;11(1):518-529. doi: 10.1159/000547202. eCollection 2025 Jan-Dec. Kidney Dis (Basel). 2025. PMID: 40787099 Free PMC article. Review.
References
-
- Sharfuddin A. A. and Molitoris B. A., “Pathophysiology of Ischemic Acute Kidney Injury,” Nature Reviews. Nephrology 7, no. 4 (2011): 189–200. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

