Efficacy and Safety of Inclisiran in Adolescents With Genetically Confirmed Homozygous Familial Hypercholesterolemia: Results From the Double-Blind, Placebo-Controlled Part of the ORION-13 Randomized Trial
- PMID: 40391436
- PMCID: PMC12180692
- DOI: 10.1161/CIRCULATIONAHA.124.073233
Efficacy and Safety of Inclisiran in Adolescents With Genetically Confirmed Homozygous Familial Hypercholesterolemia: Results From the Double-Blind, Placebo-Controlled Part of the ORION-13 Randomized Trial
Abstract
Background: Homozygous familial hypercholesterolemia (HoFH) is a genetic disease characterized by high levels of low-density lipoprotein cholesterol (LDL-C) present from birth, leading to early-onset and progressive atherosclerotic cardiovascular disease. Early treatment initiation is crucial for cardiovascular risk reduction; however, many patients do not reach LDL-C treatment goals. Inclisiran, a small interfering RNA targeting hepatic PCSK9 (proprotein convertase subtilisin/kexin type 9), is effective and well tolerated in adult patients with hyperlipidemia; however, it has not yet been studied in pediatric patients.
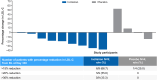
Methods: Herein we report results of the 1-year, double-blind, placebo-controlled part of the phase 3 study ORION-13 (Study to Evaluate Efficacy and Safety of Inclisiran in Adolescents With Homozygous Familial Hypercholesterolemia) in adolescents with HoFH. This 2-part multicenter study included 13 patients ≥12 to <18 years of age with a genetic diagnosis of HoFH (excluding LDL [low-density lipoprotein] receptor [LDLR] null/null genotypes) and elevated LDL-C levels (>130 mg/dL) on maximally tolerated statin treatment, with or without other lipid-lowering therapies. Eligible patients were randomized 2:1 to receive either 300 mg of inclisiran sodium or placebo, administered on days 1, 90, and 270. The primary end point was the mean percentage change in LDL-C from baseline to day 330.
Results: The mean age of patients was 14.8 years, and mean baseline LDL-C was 272 mg/dL. The placebo-adjusted mean (95% CI) percentage change in LDL-C from baseline to day 330 was -33.3% (-59.2% to -7.3%). Six of 9 (66.7%) inclisiran-treated patients (versus 1 of 4 [25%] on placebo) achieved a >15% reduction in LDL-C, and 5 of 9 (55.6%) inclisiran-treated patients (versus none on placebo) achieved a >20% reduction. The placebo-adjusted mean (95% CI) percentage change in PCSK9 from baseline to day 330 was -60.2% (-79.8% to -40.7%); corresponding changes in apolipoprotein B, non-high-density lipoprotein cholesterol, and total cholesterol were -23.0%, -32.7%, and -27.8%, respectively. No serious adverse events, treatment discontinuations because of adverse events, or deaths occurred. No new safety findings were reported.
Conclusions: In a 1-year randomized controlled study (part 1 of ORION-13), inclisiran was effective in lowering LDL-C in adolescents with HoFH and was well tolerated. These results support inclisiran as a potentially useful addition for the treatment of adolescents with HoFH and a minimum of LDLR residual activity.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04659863.
Keywords: LDL-C; adolescent; homozygous familial hypercholesterolemia; inclisiran; pediatric.
Conflict of interest statement
A.W. reports research grants from Amgen, Esperion, Novartis, Regeneron, Sanofi, Silence Therapeutics, and Ultragenyx, and consulting fees from Chiesi, Novartis, and Ultragenyx. A.L.P. reports participation on the Novartis steering committee. R.A.H. reports consulting fees from Acasti, Aegerion, Akcea/Ionis, Amgen, Arrowhead, HLS Therapeutics, Medison, Novartis, Pfizer, Regeneron, Sanofi, and Ultragenyx. E.B. reports consulting fees from Aegerion, Akcea/Ionis, Amgen, Chiesi, Ipsen, Novartis, Pfizer, Sanofi, Servier, Viatris, and Ultragenyx. A.S. is an employee of Novartis and owns Novartis shares. A.L. and Y.W. are employees of Novartis. J.D. reports consulting fees from Novartis.
Figures


References
-
- Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen-Thiessen E, et al. ; European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–2157. doi: 10.1093/eurheartj/ehu274 - PMC - PubMed
-
- Cuchel M, Raal FJ, Hegele RA, Al-Rasadi K, Arca M, Averna M, Bruckert E, Freiberger T, Gaudet D, Harada-Shiba M, et al. 2023 Update on European Atherosclerosis Society consensus statement on homozygous familial hypercholesterolaemia: new treatments and clinical guidance. Eur Heart J. 2023;44:2277–2291. doi: 10.1093/eurheartj/ehad197 - PMC - PubMed
-
- Hu P, Dharmayat KI, Stevens CAT, Sharabiani MTA, Jones RS, Watts GF, Genest J, Ray KK, Vallejo-Vaz AJ. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Circulation. 2020;141:1742–1759. doi: 10.1161/CIRCULATIONAHA.119.044795 - PubMed
-
- Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol. 2020;75:2553–2566. doi: 10.1016/j.jacc.2020.03.057 - PubMed
-
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, et al. ; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490a. doi: 10.1093/eurheartj/eht273 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

