Long-Term Safety and Efficacy of Renal Denervation: 24-Month Results From the SPYRAL HTN-ON MED Trial
- PMID: 40391448
- PMCID: PMC12244969
- DOI: 10.1161/CIRCINTERVENTIONS.125.015194
Long-Term Safety and Efficacy of Renal Denervation: 24-Month Results From the SPYRAL HTN-ON MED Trial
Abstract
Background: Six-month results from the SPYRAL HTN-ON MED trial (SPYRAL HTN-ON MED Study of Renal Denervation With the Symplicity Spyral Multi-Electrode Renal Denervation System) demonstrated that renal denervation (RDN) reduced office blood pressure (BP), and not 24-hour ambulatory systolic BP, compared with sham control in hypertensive patients. In this prespecified analysis of the ON MED trial, long-term changes in BP, antihypertensive drug use, and safety outcomes through 24 months are compared between RDN and sham control groups.
Methods: SPYRAL HTN-ON MED is a prospective, randomized, sham-controlled, blinded trial enrolling 337 patients globally from 56 clinical centers. Eligible patients had an office systolic BP of 150 to 180 mm Hg, a diastolic BP ≥90 mm Hg, and a 24-hour ambulatory systolic BP of 140 to 170 mm Hg. Patients were randomized to RDN or a sham control procedure and were prescribed a stable regimen of 1 to 3 antihypertensive medications through 6 months. After 6 months, patients and physicians were unblinded with permitted changes to antihypertensive therapy, and control patients were permitted to cross over. Crossover patients had their last observations carried forward as part of the control group. Statistical analyses were conducted on the population as randomized.
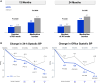
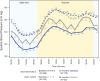
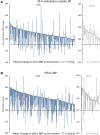
Results: At 24 months, the RDN group experienced significantly greater mean reductions in ambulatory systolic BP (-12.1±15.3 mm Hg [n=176] versus -7.0±13.1 mm Hg [n=33]; difference: -5.7 mm Hg; P=0.039) and office systolic BP (-17.4±16.1 mm Hg [n=187] versus -9.0±19.4 mm Hg [n=35]; difference: -8.7 mm Hg; P=0.0034) compared with sham controls. At 24 months, antihypertensive medications increased significantly more in the sham group (1.7 versus 2.7) compared with the RDN group (1.8 versus 2.4; P=0.046). Sensitivity analyses accounting for missing sham patient BP values due to crossover yielded consistent results in favor of RDN for 24-hour ambulatory (P=0.023) and office systolic BP (P<0.0001). Clinically adverse events were rare, with no instances of renal artery stenosis through 24 months.
Conclusions: RDN produced significantly greater ambulatory and office systolic BP reductions at 24 months compared with sham control, despite higher antihypertensive medication use in the control group.
Registration: URL: https://clinicaltrials.gov; Unique identifier: NCT02439775.
Keywords: antihypertensive agents; blood pressure; denervation; hypertension.
Conflict of interest statement
Dr Kandzari discloses receiving institutional research/grant support from Biotronik, Boston Scientific, OrbusNeich, Teleflex, Medtronic, and Ablative Solutions; he also receives personal consulting honoraria from Ablative Solutions, Medtronic, and HyperQure. Dr Mahfoud has been supported by Deutsche Forschungsgemeinschaft (SFB TRR219, Project ID 322900939) and Deutsche Herzstiftung. Saarland University has received scientific support from Ablative Solutions, Medtronic, and ReCor Medical. Until May 2024, Dr Mahfoud has received speaker honoraria/consulting fees from Ablative Solutions, AstraZeneca, Inari, Medtronic, Merck, Novartis, Philips, and ReCor Medical. Dr Townsend is a consultant for Medtronic, Axio, Regeneron, Bard, OBIO, and AstraZeneca. Royalties from UpToDate. Dr Kario receives personal fees from Medtronic, receives grants from A&D Company, JIMRO, Omron Healthcare, CureApp, Terumo, and Fukuda Denshi, receives honoraria from Otsuka Pharmaceuticals and Omron Healthcare, and participates on the advisory board of Fukuda Denshi outside the submitted work. Dr Weber has received consulting fees from Medtronic, ReCor Medical, Ablative Solutions, Johnson & Johnson, and Urovant. Dr Schmieder has received speaker and consulting honoraria from Medtronic, Recor, and Ablative Solutions. Research grants have been given to his institution from Medtronic, Recor, and Ablative Solutions. Dr Tsioufis reports institutional research/grant support from Medtronic and ReCor Medical, and personal consulting honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic, ReCor Medical, Servier, Win Medica, and ELPEN. Dr Pocock reports personal fees from Medtronic, Edwards Lifesciences, and Boston Scientific. Drs Liu and Brar are employees of Medtronic. V. DeBruin is an employee of Medtronic. Dr Böhm is supported by the Deutsche Forschungsgemeinschaft (SFB TTR219), receives personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, ReCor Medical, Servier, and Vifor.
Figures


References
-
- Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulle AM, Abera SF, Aboyans V, et al. ; GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8 - PMC - PubMed
-
- Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180 - PubMed
-
- Lauder L, Azizi M, Kirtane AJ, Böhm M, Mahfoud F. Device-based therapies for arterial hypertension. Nat Rev Cardiol. 2020;17:614–628. doi: 10.1038/s41569-020-0364-1 - PubMed
-
- Mahfoud F, Schlaich MP, Lobo MD. Device therapy of hypertension. Circ Res. 2021;128:1080–1099. doi: 10.1161/CIRCRESAHA.121.318091 - PubMed
-
- Barbato E, Azizi M, Schmieder RE, Lauder L, Böhm M, Brouwers S, Bruno RM, Dudek D, Kahan T, Kandzari DE, et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2023;44:1313–1330. doi: 10.1093/eurheartj/ehad054 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

