Seeing an Unobservable Fe(III)/Fe(IV) Redox Process of the Nonheme Iron N4Py Complex by High-Speed Surface-Enhanced Raman Spectroelectrochemistry
- PMID: 40391616
- PMCID: PMC12135866
- DOI: 10.1021/acs.inorgchem.5c01017
Seeing an Unobservable Fe(III)/Fe(IV) Redox Process of the Nonheme Iron N4Py Complex by High-Speed Surface-Enhanced Raman Spectroelectrochemistry
Abstract
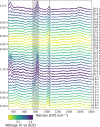
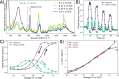
High-valent iron oxido species, central to many enzymatic and biomimetic catalyzed organic oxidative transformations, can be generated by direct electrochemical oxidation, circumventing high-energy O atom donor reagents. Electrochemical generation necessitates knowledge of the redox potentials involved, which is hindered by the lack of well-defined Fe(III)/Fe(IV) redox waves in the voltammetry of many iron-based catalysts. Hence, other approaches including chemical oxidation and bulk spectro(electro)chemical methods need to be taken. In the case of the well-studied oxidation catalyst, , where N4Py is 1,1-bis(pyridin-2-yl)-N,N-bis(pyridin-2-ylmethyl)methanamine, estimates of the Fe(III/IV) redox potentials range from 0.4 to 1.3 V vs SCE. Here, we show that electrochemical surface-enhanced Raman scattering spectroscopy reveals "hidden" redox waves, and hence redox potentials, when coupled with cyclic voltammetry. Rapid spectral acquisition (>2 Hz) of surface-enhanced Raman spectra at electrochemically roughened gold electrodes enables real-time spectral acquisition during cyclic voltammetry. We show that the Fe(III)/Fe(IV) redox potential of is close to that determined earlier by chemical redox titrations (0.85 V vs SCE). Furthermore, comproportionation and adsorption processes are shown to impact the rates of electron transfer observed, which rationalizes the absence of a distinct Fe(III)/Fe(IV) redox wave in its cyclic voltammetry.
Figures






Similar articles
-
Generation of [(N4Py)Fe(IV)═O]2+ through Heterolytic O-O Bond Cleavage in [(N4Py)Fe(II)(OOH)].Inorg Chem. 2025 May 19;64(19):9408-9417. doi: 10.1021/acs.inorgchem.4c05172. Epub 2025 May 2. Inorg Chem. 2025. PMID: 40315350 Free PMC article.
-
Nonheme FeIV═O Complexes Supported by Four Pentadentate Ligands: Reactivity toward H- and O- Atom Transfer Processes.Inorg Chem. 2023 Nov 13;62(45):18338-18356. doi: 10.1021/acs.inorgchem.3c02526. Epub 2023 Nov 1. Inorg Chem. 2023. PMID: 37913548 Free PMC article.
-
Brønsted acid-promoted C-H bond cleavage via electron transfer from toluene derivatives to a protonated nonheme iron(IV)-oxo complex with no kinetic isotope effect.J Am Chem Soc. 2013 Apr 3;135(13):5052-61. doi: 10.1021/ja311662w. Epub 2013 Mar 25. J Am Chem Soc. 2013. PMID: 23528016
-
Redox potential and C-H bond cleaving properties of a nonheme Fe(IV)=O complex in aqueous solution.J Am Chem Soc. 2010 Jun 9;132(22):7638-44. doi: 10.1021/ja909923w. J Am Chem Soc. 2010. PMID: 20476758 Free PMC article.
-
A Mononuclear Nonheme Iron(IV)-Oxo Complex of a Substituted N4Py Ligand: Effect of Ligand Field on Oxygen Atom Transfer and C-H Bond Cleavage Reactivity.Inorg Chem. 2019 Feb 4;58(3):1862-1876. doi: 10.1021/acs.inorgchem.8b02577. Epub 2019 Jan 15. Inorg Chem. 2019. PMID: 30644733
References
-
- Feig A. L., Lippard S. J.. Reactions of Non-Heme Iron(II) Centers with Dioxygen in Biology and Chemistry. Chem. Rev. 1994;94:759–805. doi: 10.1021/cr00027a011. - DOI
-
- McDonald A. R., Que L.. High-Valent Nonheme Iron-Oxo Complexes: Synthesis, Structure, and Spectroscopy. Coord. Chem. Rev. 2013;257:414–428. doi: 10.1016/j.ccr.2012.08.002. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

