Copper-Photoredox-Catalyzed C(sp3)-C(sp3) Reductive Cross-Coupling of Alkyl Bromides with BCP-Thianthrenium Reagents
- PMID: 40391618
- PMCID: PMC12281079
- DOI: 10.1002/anie.202506785
Copper-Photoredox-Catalyzed C(sp3)-C(sp3) Reductive Cross-Coupling of Alkyl Bromides with BCP-Thianthrenium Reagents
Abstract
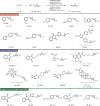
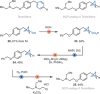
Herein, we report a reductive cross-coupling reaction of bicyclo[1.1.1]pentyl (BCP)-thianthrenium reagents and alkyl bromides. The reaction is catalyzed by a copper/photoredox catalyst system. The approach is the first example of a cross-coupling between BCP-based reagents with alkyl electrophiles.
Keywords: Bicyclopentylation; Bioisosteres; Copper catalysis; Reductive coupling; Thianthrenium salt.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Bicyclopentylation of Alcohols with Thianthrenium Reagents.J Am Chem Soc. 2023 Dec 6;145(48):25954-25961. doi: 10.1021/jacs.3c10024. Epub 2023 Nov 27. J Am Chem Soc. 2023. PMID: 38010346 Free PMC article.
-
Enabling the Use of Alkyl Thianthrenium Salts in Cross-Coupling Reactions by Copper Catalysis.Angew Chem Int Ed Engl. 2021 Sep 27;60(40):21756-21760. doi: 10.1002/anie.202109723. Epub 2021 Aug 26. Angew Chem Int Ed Engl. 2021. PMID: 34378844
-
Dual Nickel- and Photoredox-Catalyzed Asymmetric Reductive Cross-Couplings: Just a Change of the Reduction System?Acc Chem Res. 2024 Jul 16;57(14):1997-2011. doi: 10.1021/acs.accounts.4c00309. Epub 2024 Jul 3. Acc Chem Res. 2024. PMID: 38961540
-
Ni-Catalyzed C-C Couplings Using Alkyl Electrophiles.Top Curr Chem (Cham). 2016 Oct;374(5):66. doi: 10.1007/s41061-016-0067-6. Epub 2016 Aug 31. Top Curr Chem (Cham). 2016. PMID: 27580894 Review.
-
Dual Copper/Photoredox Catalyzed C-H Functionalizations.Chem Asian J. 2025 Jul 24:e00692. doi: 10.1002/asia.202500692. Online ahead of print. Chem Asian J. 2025. PMID: 40703043 Review.
References
-
- Stepan A. F., Subramanyam C., Efremov I. V., Dutra J. K., O'Sullivan T. J., DiRico K. J., McDonald W. S., Won A., Dorff P. H., Nolan C. E., Becker S. L., Pustilnik L. R., Riddell D. R., Kauffman G. W., Kormos B. L., Zhang L., Lu Y., Capetta S. H., Green M. E., Karki K., Sibley E., Atchison K. P., Hallgren A. J., Oborski C. E., Robshaw A. E., Sneed B., O'Donnell C. J., J. Med. Chem. 2012, 55, 3414–3424. - PubMed
-
- Auberson Y. P., Brocklehurst C., Furegati M., Fessard T. C., Koch G., Decker A., La Vecchia L., Briard E., ChemMedChem 2017, 12, 590–598. - PubMed
-
- Nilova A., Campeau L.‐C., Sherer E. C., Stuart D. R., J. Med. Chem. 2020, 63, 13389–13396. - PubMed
LinkOut - more resources
Full Text Sources

