The Immune Microenvironment: New Therapeutic Implications in Organ Fibrosis
- PMID: 40391706
- PMCID: PMC12376546
- DOI: 10.1002/advs.202505067
The Immune Microenvironment: New Therapeutic Implications in Organ Fibrosis
Abstract
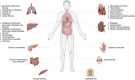
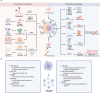
Fibrosis, characterized by abnormal deposition of structural proteins, is a major cause of tissue dysfunction in chronic diseases. The disease burden associated with progressive fibrosis is substantial, and currently approved drugs are unable to effectively reverse it. Immune cells are increasingly recognized as crucial regulators in the pathological process of fibrosis by releasing effector molecules, such as cytokines, chemokines, extracellular vesicles, metabolites, proteases, or intercellular contact. Therefore, targeting the immune microenvironment can be a potential strategy for fibrosis reduction and reversion. This review summarizes the recent advances in the understanding of the immune microenvironment in fibrosis including phenotypic and functional transformations of immune cells and the interaction of immune cells with other cells. The novel opportunities for the discovery and development of drugs for immune microenvironment remodeling and their associated challenges are also discussed.
Keywords: immune microenvironment; organ fibrosis; therapeutic targets.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Electrophoresis.2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36251838 Free Books & Documents.
References
-
- Zhao X., Kwan J. Y. Y., Yip K., Liu P. P., Liu F. F., Nat. Rev. Drug Discovery 2020, 19, 57. - PubMed
Publication types
MeSH terms
Grants and funding
- 82025007/National Natural Science Foundation of China
- U24A20676/National Natural Science Foundation of China
- 82170874/National Natural Science Foundation of China
- 82270923/National Natural Science Foundation of China
- 23NSFTD0067/Innovation Group Project from Science & Technology Department of Sichuan Province
LinkOut - more resources
Full Text Sources
